| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Email: |
marketing@energy-chemical.com |
| Products Intro: |
Product Name:Amosulalol Hydrochloride
CAS:70958-86-0
Purity:NULL Package:2.5mg;25mg Remarks:NULL
|
| Company Name: |
CLEARSYNTH LABS LTD.
|
| Tel: |
+91-22-45045900 |
| Email: |
sales@clearsynth.com |
| Products Intro: |
Product Name:Amosulalol
CAS:70958-86-0
|
| Company Name: |
Toronto Research Chemicals
|
| Tel: |
+1 (416) 665-9696 |
| Email: |
info@trc-canada.com |
| Products Intro: |
Product Name:Amosulalol Hydrochloride
CAS:70958-86-0
Package:5mg
|
|
| | YM 09538 Basic information |
| Product Name: | YM 09538 | | Synonyms: | Benzenesulfonamide, 5-[1-hydroxy-2-[[2-(2-methoxyphenoxy)ethyl]amino]ethyl]-2-methyl-, monohydrochloride;Lowgan;YM 09538;AMOSULALOLHYOROCHLORIDE;5-[1-Hydroxy-2-[[2-(2-methoxyphenoxy)ethyl]amino]ethyl]-2-methyl-benzenesulfonamide monohydrochloride;Rowgan | | CAS: | 70958-86-0 | | MF: | C18H24N2O5S.HCl | | MW: | 0 | | EINECS: | | | Product Categories: | | | Mol File: | 70958-86-0.mol | 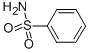 |
| | YM 09538 Chemical Properties |
| | YM 09538 Usage And Synthesis |
| Originator | Amosulalol
hydrochloride,Yamanouchi | | Uses | Amosulalol Hydrochloride is used in studies to evaluate the effect of α1-blocker doxazosin on diabetic nephropathy -comparison with α,β -blockers. | | Definition | ChEBI: Amosulalol hydrochloride is a sulfonamide. | | Manufacturing Process | A mixture of N-benzyl-2-(2-methoxyphenoxy)ethylamine, methyl ethyl ketone,
and 5-bromoacetyl-2-methylbenzenesulfonamide was refluxed with stirring.
After cooling the reaction mixture, ethyl ketone was distilled off under reduced
pressure and the residue formed was dissolved in benzene. Then, ether was
added to the solution and after removing the hydrobromide of N-benzyl-2-(2-
methoxyphenoxy)ethylamine precipitated, the solvent was distilled off under
reduced pressure to provide a viscous oily product.
The viscous oily product was dissolved in ethanol and after adding to the
solution an excess amount of sodium borohydride, the mixture was stirred at
room temperature followed by distilling off ethanol under reduced pressure.
The residue was dissolved in ethyl acetate and the ethyl acetate layer
recovered was washed with water, dried over anhydrous sodium sulfate, and
concentrated under reduced pressure to provide a viscous oily product. The
product was subjected to a silica gel column chromatography and eluted using
benzene and then a mixture of benzene and ethyl acetate of 10:1 by volume
ratio to provide 5-{1-hydroxy-2-[N-benzyl-2-(2-
methoxyphenoxy)ethylamino]ethyl}-2-methyl-benzenesulfonamide as a
viscous oily product.
In 200 ml of methanol was dissolved 20.0 g of 5-{1-hydroxy-2-[N-benzyl-2-
(2-methoxyphenoxy)ethylamino]ethyl}-2-methyl-benzenesulfonamide. After
adding thereto 20 ml of ethanol containing about 10% hydrogen chloride and
1.0 g of 10% palladium charcoal, the mixture was shaken in hydrogen gas
stream. When the absorption of hydrogen stopped, the catalyst was filtered
away and the filtrate was distilled off under reduced pressure. The residue
was dissolved in 100 ml of ethanol while it was hot and the solution was
allowed to stand overnight in ice chamber, whereby 12.8 g of the α-type
crystals of 5-{1-hydroxy-2-[2-(2-methoxyphenoxy)ethylamino]ethyl}-2-
methylbenzenesulfonamide were obtained as the colorless crystals, melting
point 169°-171°C.
In practice it is usually used as monohydrochloride | | Therapeutic Function | Alpha-adrenergic blocker, Beta-adrenergic blocker,
Antihypertensive |
| | YM 09538 Preparation Products And Raw materials |
|