| Company Name: |
ShangHai Anpel Co, Ltd.
|
| Tel: |
18501792038; 18501792038 |
| Email: |
shanpel@anpel.com.cn |
| Products Intro: |
Product Name:Perchlorate standard solution,ClO4-(NaClO4)
CAS:14797-73-0
Purity:1000ppm Package:1000mg/L于水(以高氯酸根计),2ml
|
| Company Name: |
CLEARSYNTH LABS LTD.
|
| Tel: |
+91-22-45045900 |
| Email: |
sales@clearsynth.com |
| Products Intro: |
Product Name:Perchlorate
CAS:14797-73-0
|
| Company Name: |
AccuStandard Inc
|
| Tel: |
(800) 442-5290 |
| Email: |
info@bester.nl |
| Products Intro: |
Product Name:PT NELAC WS Perchlorate Standard
CAS:14797-73-0
Purity:10 - 50 in μg/L in Water Package:$47.00/20mL
|
|
| | PERCHLORATE Basic information |
| Product Name: | PERCHLORATE | | Synonyms: | Hyperchloric acid anion;Hyperchloric acid ion;Perchlorate ion;Perchlorate ion(1-) | | CAS: | 14797-73-0 | | MF: | ClO4 | | MW: | 99.45 | | EINECS: | | | Product Categories: | | | Mol File: | 14797-73-0.mol | 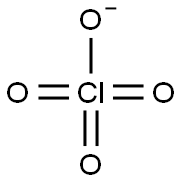 |
| | PERCHLORATE Chemical Properties |
| | PERCHLORATE Usage And Synthesis |
| Description | Concern about environmental perchlorate exposure centers on
its inhibition of iodide uptake into the thyroid. Decreased
iodine intake may decrease thyroid hormone production. In
the last few years, perchlorate has become a major inorganic
contaminant in drinking water and has been detected in
a number of public drinking water systems throughout the
Unites States. In 1998, the US EPA added perchlorate to the
Drinking Water Candidate Contaminant List. | | Uses | The primary source of perchlorate is the ammonium salt.
Ammonium perchlorate is the oxidizer ingredient in solid
propellant mixtures for rockets, missiles, and munitions. Other
uses of perchlorate salts include medicine, matches, metal
cation chemistry, and pyrotechnics (illuminating and signaling
flares, colored and white smoke generators, tracers, incendiary
delays, fuses, photo-flash compounds, and fireworks).
Perchlorate is also found in lubricating oils, finished leather,
fabric fixer, dyes, electroplating, aluminum refining, manufacture
of rubber, paint, and enamel production, as an additive in
cattle feed and magnesium batteries, and as a component of
automobile airbag inflators. | | Uses | Perchlorate is a soluble oxychloro anion most commonly used as a solid salt in the form of ammonium perchlorate, potassium perchlorate, lithium perchlorate, or sodium per- chlorate, all of which are highly soluble. Ammonium perchlorate is the most widely used perchlorate compound. In their pure forms, these salts are white or colorless crystals or powders. Perchlorate salts dissolve in water and readily move from surface to groundwater. Perchlorate is known to originate from both natural and man-made sources.
The most common uses for ammonium perchlorate are in explosives, military munitions, and rocket propellants. In addition, perchlorate salts are used in a wide range of nonmilitary applications, including pyrotechnics and fireworks, blasting agents, solid rocket fuel, matches, lubricating oils, nuclear reactors, air bags, and certain types of fertilizers. Improper storage and disposal related to these uses is the most typical route for perchlorate to enter into the environment. Monitoring data show that more than 4% of public water systems, serving between 5 million and 17 million people, have detected perchlorate in their finished water. | | Definition | ChEBI: Perchlorate is a monovalent inorganic anion obtained by deprotonation of perchloric acid. It is a monovalent inorganic anion and a chlorine oxoanion. It is a conjugate base of a perchloric acid. | | General Description | Crystalline or powdered solids. May explode under exposure to heat or fire. If available, obtain the technical name from the shipping papers and contact CHEMTREC, 800-424-9300 for specific response information. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | PERCHLORATE is a strong oxidizing agents. May be self-reactive (e.g., ammonium perchlorate, glycol perchlorate) and liable to violent decomposition. The violence of decomposition of some perchlorates exceeds that of nitroglycerine. Noncombustible but able to accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, then an explosion may result. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Environmental Fate | Environmental Persistency (Degradation and Speciation):
Perchlorate salts such as ammonium perchlorate are
expected to exist as a solid aerosol or be absorbed to suspended
particulate matter. Therefore, removal from the
atmosphere is expected to occur by both wet and dry
depositions. Perchlorates are not expected to undergo direct
photolysis in air. If released in soil, the perchlorate ion is
only weakly absorbed to mineral surfaces of moderate ionic
strength. The ion exhibits high aqueous solubility, and
together these properties contribute to its ability to readily
migrate in groundwater systems. The ion is not expected to
volatilize from soil to the atmosphere because perchlorates
exhibit very low vapor pressures. If released to water,
ammonium perchlorate readily dissolves and dissociates to
the perchlorate ion. Perchlorate is an ion; therefore, volatilization
from water surfaces is not expected to be an
important fate process. Hydrolysis does not occur for
inorganic salts such as ammonium perchlorate, which
ionizes in aqueous solution.
Handling and storage: Keep the container tightly closed. Keep
the container in a cool, well-ventilated area, separate from
acids, alkalies, reducing agents, and combustibles.
Bioaccumulation and biomagnifications: Significant decomposition
of perchlorate does not occur in nature, making
human interventions necessary for efficient environmental
cleanup. | | Toxicity evaluation | The perchlorate ion, because of its similarity to iodide in ionic
size and charge,competes with iodide for uptake into the thyroid
gland. At therapeutic dosage levels (100–1000 mg day°1), this
competitive inhibition results in reduced production of the
thyroid hormones T3 and T4 and a consequent increase in TSH
via a negative feedback loop involving the thyroid, pituitary, and
hypothalamus.
The competitive inhibition of iodide uptake is the only direct
perchlorate effect on the thyroid, leading to a reversible chemical-
induced iodine deficiency. Inhibition of iodide uptake in
the thyroid of adult male rats dosed intravenously was detected
at a dose as low as 0.01 mg kg-1 perchlorate. Perchlorate has
not been shown to produce iodine deficiency in humans.
The effects of perchlorate on iodine availability should be
interpreted in the context of other sodium iodide symporter
inhibitors. These include thiocyanate, a metabolite of cyanide
that is produced as a byproduct of cigarette smoke and found in
a large variety of foods, and nitrate, which is produced naturally.
Comparatively, perchlorate is a very potent inhibitor of
the sodium iodide symporter; its effects are 15-fold greater than
thiocyanate, 30-fold compared with iodide, and 240-fold
compared with nitrate. |
| | PERCHLORATE Preparation Products And Raw materials |
|