|
|
| | 4-Biphenylyl Trifluoromethanesulfonate Basic information |
| Product Name: | 4-Biphenylyl Trifluoromethanesulfonate | | Synonyms: | 4-Biphenylyl Trifluoromethanesulfonate;4-Phenylphenyl triflate;Methanesulfonic acid, 1,1,1-trifluoro-, [1,1'-biphenyl]-4-yl ester;4-Biphenylyl Triflate;(4-phenylphenyl) trifluoromethanesulfonate;(4-phenylphenyl) trifluoromethanesulfonate - [P83658] | | CAS: | 17763-78-9 | | MF: | C13H9F3O3S | | MW: | 302.27 | | EINECS: | | | Product Categories: | | | Mol File: | 17763-78-9.mol | 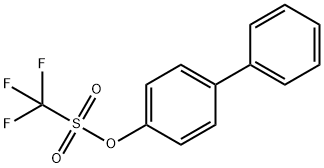 |
| | 4-Biphenylyl Trifluoromethanesulfonate Chemical Properties |
| Melting point | 52.0 to 56.0 °C | | Boiling point | 365.4±42.0 °C(Predicted) | | density | 1.398±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | soluble in Chloroform | | form | powder to crystal | | color | White to Light yellow | | InChI | InChI=1S/C13H9F3O3S/c14-13(15,16)20(17,18)19-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H | | InChIKey | RJFYGCJTUUXOOF-UHFFFAOYSA-N | | SMILES | C(F)(F)(F)S(OC1=CC=C(C2=CC=CC=C2)C=C1)(=O)=O |
| | 4-Biphenylyl Trifluoromethanesulfonate Usage And Synthesis |
| Synthesis | General procedure for the synthesis of 4-triphenyltrifluoromethanesulfonate from trifluoromethanesulfonic anhydride and p-hydroxybiphenyl: p-hydroxybiphenyl (1.0 eq.) was added to a flame-dried 25 mL round-bottomed flask fitted with a rubber septum and a magnetic stir bar. The system was evacuated and backfilled with nitrogen and the process was repeated three times. Anhydrous dichloromethane (0.7 M) and distilled pyridine (4.2 eq.) were added via syringe and the stirred solution was cooled in an ice bath for 10 min. Trifluoromethanesulfonic anhydride (1.4 eq.) was added slowly dropwise over 5 min. The reaction mixture was allowed to warm slowly to room temperature. After the reaction had proceeded for 10-14 hours, the reaction mixture was diluted with ether (30 mL) and the reaction was quenched with 1 M aqueous hydrochloric acid (30 mL). The organic layer was separated, washed sequentially with water (2 x 25 mL) and brine (25 mL), then dried with anhydrous magnesium sulfate, filtered, and concentrated by rotary evaporation. Finally, the target product, 4-triphenylphenyl trifluoromethanesulfonate, was purified by fast chromatography (elution gradient: 2-20% ethyl acetate/hexane, 45x column volume). | | References | [1] Tetrahedron, 2018, vol. 74, # 26, p. 3314 - 3317
[2] Chemistry - A European Journal, 2007, vol. 13, # 5, p. 1432 - 1441
[3] Angewandte Chemie - International Edition, 2018, vol. 57, # 34, p. 11045 - 11049
[4] Angew. Chem., 2018, vol. 130, # 34, p. 11211 - 11215,5
[5] Angewandte Chemie - International Edition, 2017, vol. 56, # 49, p. 15693 - 15697 |
| | 4-Biphenylyl Trifluoromethanesulfonate Preparation Products And Raw materials |
|