| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | alsactide Basic information |
| Product Name: | alsactide | | Synonyms: | alsactide;ACTH 4-amino-n-butylamide (1-17), Ala(1)-Lys(17)-;1-β-Alanine-17-[N-(4-aminobutyl)-L-lysinamide]-α1-17-corticotropin;βAla-L-Tyr-L-Ser-L-Met-L-Glu-L-His-L-Phe-L-Arg-L-Trp-Gly-L-Lys-L-Pro-L-Val-Gly-L-Lys-L-Lys-N6-(4-Aminobutyl)-L-Lys-NH2;α1-17-Corticotropin, 1-β-alanine-17-[N-(4-aminobutyl)-L-lysinamide]- (9CI) | | CAS: | 34765-96-3 | | MF: | C99H155N29O21S | | MW: | 2119.54 | | EINECS: | 252-203-0 | | Product Categories: | | | Mol File: | 34765-96-3.mol | 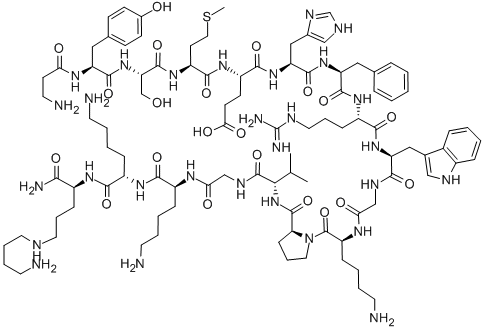 |
| | alsactide Chemical Properties |
| density | 1.43±0.1 g/cm3(Predicted) |
| | alsactide Usage And Synthesis |
| Originator | Alsactide,ZYF Pharm Chemical | | Manufacturing Process | Abbreviations:
Boc - tert-butoxycarbonyl; TCP - 2,4,5-trichlorophenyl; Z - carbobenzyloxy.
(a) β-Ala-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Lys-
NH-(CH2)4-NH2-acetate, aq.
Z-Lys(Boc)-NH-(CH2)4-NH-Boc: 7.0 g (31 mmols) of Boc-NH-(CH2)4-NH2-HCl
and 4.2 ml (30 mmols) of triethylamine in 100 ml of dimethylformamide are
stirred for 20 hours together with 16.8 g (30 mmols) of Z-Lys(Boc) OTCP. The
product is filtered off the triethylammonium chloride and the filtrate is
evaporated to dryness in vacuo. The residue is dissolved in ethyl acetate,
thoroughly shaken at 0°C with 2 N citric acid, 1 N bicarbonate and H2O and
dried over sodium sulfate. After distilling off the solvents, the residue is
recrystallized two times from isopropanol/ether. Yield: 14.1 g (83%), M.P.
83°-85°C.
(b) H-Lys(Boc)-NH-(CH2)4-NH-Boc-tosylate: 11.4 g of the Z-compound
prepared according to (a) are hydrogenated in the presence of Pd in 80 ml of
methanol with, while adding methanolic toluene-sulfonic acid at pH 5. After
the reaction is complete, the product is filtered to remove the catalyst, the
methanol is distilled off in vacuo and the residue is triturated with ether. For
purification purposes it is dissolved in warm isopropanol and precipitated with
ether. Yield: 10.3 g (88%).
(c) Z-Lys(Boc)-Pro-Val-Gly-Lys(Boc)-Lys(Boc)-Lys(Boc)-NH-(CH2)4-NH-Boc:
10.9 g (10 mmols) of Z-Lys(Boc)-Pro-Val-Gly-Lys(Boc)-OH and 5.89 g (10
mmols) of Boc-NH-(CH2)4-NH2-tosylate in 100 ml of dimethylformamide are
combined with 12.8 ml (10 mmols) of N-ethylmorpholine and 2.7 g (20
mmols) of 1-hydroxybenzotriazole. 2.2 g of dicyclohexylcarbodiimide (11
mmols) are added at -10°C. The whole is then allowed to come to room
temperature. The stirring is continued for 3 hours, the solvent is distilled off in
vacuo. The residue is digested with 1 N bicarbonate and water and, after
drying, recrystallized from acetonitrile. Yield: 10.6 g (74.2%). M.P. 150°-
155°C (while foaming). [α]D
20: -24.0° (c=1 in dimethylformamide).
(d) H-Lys(Boc)-Pro-Val-Gly-Lys(Boc)-Lys(Boc)-Lys(Boc)-NH-(CH2)4-NH-Boctosylate-
dihydrate: 15.1 g (10 mmols) of the Z-compound prepared according
to (c) are catalytically hydrogenated in the presence of Pd in 300 ml of
methanol, a pH of 5 being maintained by simultaneously adding methanolic
toluene-sulfonic acid. After complete reaction, the methanol is distilled off and
the residue is reprecipitated from pyridine/ ether and methanol/water. The oil,
which first precipitates becomes solid after a short time. Yield: 12.1 g
(77.5%).
(e) 1.65 g (1.1 mmols) of Boc-β-Ala-Tyr-Ser-Met-Glu-(OBut)-His-Phe-Arg-Trp-
Gly-OH·4H2O and 1.56 g (1 mmol) of H-Lys(Boc)-Pro-Val-Gly-Lys(Boc)-Lys-
(Boc)-Lys(Boc)-NH-(CH2)4-NH-Boc-tosylate-dihydrate are dissolved together
with 540 mg (4 mmols) of 1-hydroxybenzotriazole in 30 ml of
dimethylformamide. A solution of 1.25 g (6 mmols) of
dicyclohexylcarbodiimide in 4 ml of dimethylformamide is prepared and 1/3 of
this solution is added to the above solution. The whole is stirred for 1 hour,
then another third is added and, after a further hour, the last third is fed in.
After a further 2 to 3 hours of stirring, the reaction product is precipitated
with ether. Yield: 3.1 g.
Without further purification, the compound is freed from the protective groups
by standing for 1 hour in 80-90% trifluoroacetic acid containing some
thioglycolic acid, and is subsequently precipitated by adding 150 ml of ether.
Yield: 3.06 g of crude peptide-trifluoroacetate. After purification on
carboxymethyl cellulose, 1.45 g of the chromatographically pure peptide are
obtained in the form of acetate. [α]D
20:-68.6°-2° (c=0.5 in 1% acetic acid).
Amino acid analysis:
Ser0.86Glu0.99Pro0.97Gly2.00Val1.03Met0.98Tyr0.02Phe1.00βAla1.01Lys4.00His1.02Arg0.94. | | Therapeutic Function | Adrenocorticotropin |
| | alsactide Preparation Products And Raw materials |
|