| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Moxestrol;Moxestrol
CAS:34816-55-2
Purity:98% Package:5 mg
|
Moxestrol manufacturers
- MOXESTROL USP/EP/BP
-

- $1.10 / 1g
-
2021-06-04
- CAS:
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | Moxestrol Basic information |
| Product Name: | Moxestrol | | Synonyms: | (17R)-11β-Methoxy-19-norpregna-1,3,5(10)-trien-20-yne-3,17-diol;RU-2858;Surestryl;MOXESTROL USP/EP/BP;19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, 11-methoxy-, (11β,17α)-;1, 3, 5(10)-ESTRATRIEN-17α-ETHYNYL-3, 11β, 17β-TRIOL 11-METHYL ETHER | | CAS: | 34816-55-2 | | MF: | C21H26O3 | | MW: | 326.43 | | EINECS: | | | Product Categories: | | | Mol File: | 34816-55-2.mol | 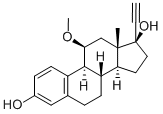 |
| | Moxestrol Chemical Properties |
| Melting point | 280° | | alpha | D20 +29° (c = 0.6 in ethanol) | | Boiling point | 404.46°C (rough estimate) | | density | 1.1348 (rough estimate) | | refractive index | 1.4700 (estimate) | | pka | 10.16±0.70(Predicted) |
| | Moxestrol Usage And Synthesis |
| Originator | Surestryl,Roussel,France,1974 | | Definition | ChEBI: Moxestrol is a 3-hydroxy steroid. | | Manufacturing Process | (A) Preparation of 11β-Methoxy-?4,9-Estradiene-3,17-Dione: 0.5 g of ?4,9-
estradiene-11β-ol-3,17-dione were dissolved at room temperature in 25 cc of
methylene chloride containing 2% of methanol and after 5 mg of p-toluene�sulfonic acid were added, the reaction mixture was agitated for several
minutes. Then the reaction mixture was poured into ice water, washed with
water until the wash waters were neutral, and distilled to dryness under
vacuum. The resulting residue was crystallized from ethyl ether to obtain 0.46
g of 11β-methoxy-?4,9-estradiene-3,17-dione having a MP of 140°C.
(B) Preparation of 11β-Methoxy-?1,3,5(10)-Estradiene-3-ol-17-one: 12.3 g of
11β-methoxy-?4,9-estradiene-3,17-dionewere dissolved in 1,230 cc of
methanol and then, under an atmosphere of nitrogen, 7.38 g of palladium
hydroxide were added and the mixture was held at reflux for one hour under
agitation and a nitrogen atmosphere. Then the reaction mixture was cooled to
30°C, filtered, vacuum filtered and washed with methanol. The methanolic
solutions were concentrated to about 50 cc, allowed to stand overnight at
room temperature and filtered. The precipitate formed was triturated in
methanol and dried at 80°C to obtain 10.74 g (yield = 87.5%) of 11β-
methoxy-?1,3,5(10)-estradiene-3-ol-17-one having a MP of 264°C.
(C)Preparation of 11β-Methoxy-17α-Ethynyl-?1,3,5(10)-Estradiene-3,17β-Diol:
Under agitation and an atmosphere of nitrogen, 12 g of potassium were
heated at 80°C in 180 cc of tertiary-amyl alcohol. The mixture was agitated
for 30 minutes, cooled to 20°C and after 60 cc of dioxane were added thereto,
a stream of acetylene was allowed to bubble through the mixture for one hour and fifteen minutes. Then a solution of 3 g of 11β-methoxy-?1,3,5(10)-
estradiene-3-ol-17-one in 50 cc of dioxane was added and the mixture was
agitated for 4 hours while continuing the passage of acetylene at room
temperature. Thereafter, 50 cc of a 50% aqueous acetic acid solution was
added and the mixture was poured into water and extracted with ether. The
organic phases were washed first with an aqueous solution containing 10% of
neutral sodium carbonate, then with water until the wash waters were neutral,
dried over sodium sulfate and concentrated under vacuum until crystallization
started. The reaction mixture was iced for one hour, vacuum filtered and the
precipitate dried under vacuum to obtain 3.8 g of the raw 17α-ethynyl
derivative, which was purified by dissolution in ethyl acetate at reflux and by
icing to obtain 2.33 g (yield = 77%) of 11β-methoxy-17α-ethynyl-?1,3,5(10)-
estradiene-3,17β-diol, having a MP of 280°C. | | Therapeutic Function | Estrogen |
| | Moxestrol Preparation Products And Raw materials |
|