|
ChemicalBook Optimization Suppliers |
|
| | グリピジド Usage And Synthesis |
| 外観 | 白色~ほとんど白色粉末~結晶 | | 効能 | 糖尿病治療薬, 血糖降下薬, スルホニル尿素受容体作動薬 | | 化学的特性 | Crystalline Solid | | Originator | Minidiab,Carlo Erba,Italy,1973 | | 使用 | Labelled Glipizide . A sulfonylurea hypoglycemic agent. Used as an antidiabetic.;Labeled Glipizide, intended for use as an internal standard for the quantification of Glipizide by GC- or LC-mass spectrometry. | | 使用 | A hypoglycemic agent that enhances insulin secretion. | | 使用 | sweetener, treatment of portoencephalopathy | | 定義 | ChEBI: An N-sulfonylurea that is glyburide in which the (5-chloro-2-methoxybenzoyl group is replaced by a (5-methylpyrazin-2-yl)carbonyl group. An oral hypoglycemic agent, it is used in the treatment of type 2 diabetes mellitus. | | Manufacturing Process | 5-Methyl pyrazine-2-carboxylic acid is refluxed with thionyl chloride in
anhydrous benzene for approximately 12 hours. Benzene and thionyl chloride
excess is removed by distillation. Then some anhydrous dioxane is added and
this acid chloride solution is allowed to drop into p-(β-aminoethyl)-
benzenesulfonamide suspension in dioxane and anhydrous pyridine. The
resulting mixture is then refluxed for 3 hours. Dioxane is removed by
distillation and then the residue is washed with water and acetic acid. The raw
acylated sulfonamide is then filtered and crystallized from 95% ethanol, thus
obtaining a product of MP 200° to 203°C.
This product is then reacted with cyclohexyl isocyanate to give glipizide. | | brand name | Glucotrol (Pfizer). | | Therapeutic Function | Oral hypoglycemic | | 一般的な説明 | Glipizide is N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-5-methyl-2-pyrazinecarboxamide;this compound can also be named as the urea—seepreceding discussion (Glucotrol, generic). In the UnitedStates, combinations are available with metformin (Metaglip,generic; tablets, mg glipizide/mg metformin as hydrochloride:2.5/250, 2.5/500, 5/500). Extended-release tablets are available(Glucotrol XL, generic). The pyrazine moiety within thisstructure renders the molecule significantly more hydrophilicthan the similar molecule glyburide, albeit also moderatelyless potent on a dosage as well as target-level basis. | | 一般的な説明 | Structurally, glipizide, 1-cyclohexyl-3-[[p-[2(methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea(Glucotrol), is a cyclohexylsulfonylurea analog similar toacetohexamide and glyburide. The drug is absorbed rapidlyon oral administration. Its serum half-life is 2 to 4 hours, andit has a hypoglycemic effect that ranges from 12 to 24 hours. | | 一般的な説明 | Glipizide, 1-cyclohexyl-3-[[p-(2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea(Glucotrol), is an off-white, odorless powder with a pKa of5.9. It is insoluble in water and alcohols, but soluble in 0.1 NNaOH. Even though on a weight basis, it is approximately100 times more potent than tolbutamide, the maximal hypoglycemiceffects of these two agents are similar. It is rapidlyabsorbed on oral administration, with a serum half-life of 2 to4 hours, whereas the hypoglycemic effects range from 12 to24 hours. Metabolism of glipizide is generally through oxidationof the cyclohexane ring to the p-hydroxy and m-hydroxymetabolites. A minor metabolite that occurs involves theN-acetyl derivative, which results from the acetylation of theprimary amine following hydrolysis of the amide system byamidase enzymes. | | Biochem/physiol Actions | Potassium inwardly-rectifying channel, subfamily J, member 1 (KCNJ1) plays a vital role in potassium balance. It is an ATP-dependent K+?channel blocker. The encoded protein is liable for the elimination of potassium in exchange for the absorption of sodium by the epithelial sodium channel (ENaC). Mutation in KCNJ1 is linked with several diseases, such as, antenatal Bartter syndrome and diabetes. Glipizide helps to repress the development of tumors and metastasis by preventing angiogenesis. | | 臨床応用 | Non-insulin dependent diabetes mellitus | | 合成 | Glipizide, 1-cyclohexyl-3-[[p-[2-(5-methylpyrazincarboxamido)ethyl]phenyl]
sulfonyl]urea (26.2.13), differs from glyburide in the structure of the amide region of the
molecule, in which the 2-methoxy-5-chlorobenzoic acid part is replaced with 6-
methylpyrazincarboxylic acid. It is also synthesized by a synthesis alternative to those
described above. In the given scheme, 6-methylpyrazincarboxylic acid is initially reacted
with thionyl chloride, resulting in the corresponding chloride, which undergoes further
action with 4-(2-aminoethyl)benzenesulfonamide, forming the corresponding amide
26.2.12. The resulting sulfonamide is reacted in a traditional scheme with cyclohexylisocyanate,
forming the desired glipizide (26.2.13). 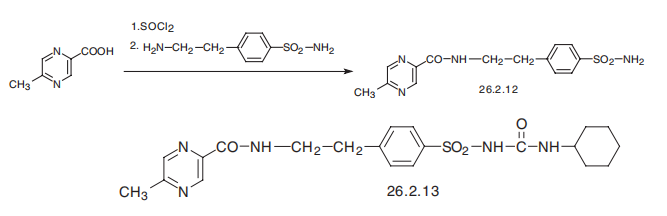
| | Veterinary Drugs and Treatments | Glipizide may be of benefit in treating cats with type II diabetes if
they have a population of functioning beta cells. It has been suggested
that there are two situations when glipizide can be recommended,
1) If an owner refuses to consider using insulin usually
due to a fear of needles, and 2) the cat appears to be relatively well
controlled on quite small doses of insulin and the owner would
strongly prefer to no longer give insulin (Feldman 2005b).
While glipizide potentially could be useful in treating canine patients
with type II or III diabetes, however, by the time dogs present
with hyperglycemia, they are absolutely or relatively insulinopenic
and glipizide would unlikely be effective. | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs.
Antibacterials: effects enhanced by chloramphenicol,
sulphonamides, tetracyclines and trimethoprim;
effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by
coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole,
posaconazole and miconazole and possibly
voriconazole - avoid with miconazole.
Ciclosporin: may increase ciclosporin levels.
Lipid-regulating drugs: possibly additive
hypoglycaemic effect with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas. | | 代謝 | The metabolism of glipizide is extensive and occurs
mainly in the liver. The primary metabolites are inactive
hydroxylation products and polar conjugates and are
excreted mainly in the urine. |
|