|
ChemicalBook Optimization Suppliers |
|
| 融点 | 98 °C | | 沸点 | 492.8±45.0 °C(Predicted) | | 比重(密度) | 1.161±0.06 g/cm3(Predicted) | | 貯蔵温度 | 2-8°C | | 溶解性 | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 4.3 mg/mL | | 外見 | solid | | 酸解離定数(Pka) | pKa 5.3 (Uncertain) | | 色 | white | | 水溶解度 | 0.2g/L(25 ºC) | | 極大吸収波長 (λmax) | 254nm(HCl aq.)(lit.) | | Merck | 14,9780 | | InChI | InChI=1S/C17H20N2O2/c1-2-19(12-14-8-10-18-11-9-14)17(21)16(13-20)15-6-4-3-5-7-15/h3-11,16,20H,2,12-13H2,1H3 | | InChIKey | BGDKAVGWHJFAGW-UHFFFAOYSA-N | | SMILES | C(CO)(C(=O)N(CC)CC1=CC=NC=C1)C1C=CC=CC=1 | | CAS データベース | 1508-75-4(CAS DataBase Reference) | | NISTの化学物質情報 | Tropicamide(1508-75-4) |
| | トロピカミド Usage And Synthesis |
| 外観 | 白色~ほとんど白色粉末~結晶 | | 効能 | 散瞳薬, ムスカリン受容体拮抗薬 | | 商品名 | ミドリン (参天製薬) | | 化学的特性 | Crystalline Solid | | Originator | Mydriacyl,Alcon,US,1959 | | 使用 | Indicated to induce mydriasis (dilation of the pupil) and cycloplegia (paralysis of the ciliary muscle of the eye) in diagnostic procedures, such as measurement of refractive errors and examination of the fundus of the eye. | | 使用 | Ophthalmic anticholinergic. Mydriatic | | 使用 | Tropicamide, like cyclopentolate, is used in ophthalmoscopy for reaching pre-operational
mydraises and for testing narrow-angle glaucoma. | | 使用 | antidepressant, nutrient; LD50(rat) 1634 mg/kg ip | | 定義 | ChEBI: Tropicamide is a member of acetamides. | | Manufacturing Process | A solution of 82 parts by weight of γ-chloromethyl-pyridine-hydrochloride in 60
parts of water is added dropwise, at 0° to 5°C, to 250 parts by weight of a
50% aqueous ethyl amine solution. The mixture is stirred for 1 hour at 60°C,
whereupon it is cooled down and separated in the cold with solid potassium
hydroxide. The oil formed is separated off, dried over potassium hydroxide
and distilled. The ethyl-(γ-picolyl)-amine formed boils over at 103° to 104°C
under a pressure of 13 mm Hg. Its dihydrochloride melts at 198° to 200°C.
To a mixture of 48.7 parts by weight of ethyl-(γ-picolyl)-amine and 36 parts
by weight of dry pyridine in 220 parts by weight of dry chloroform is slowly
added, while stirring and cooling with ice water, crude acetyltropic acid
chloride prepared from 60 parts by weight of tropic acid. To complete the
reaction, the mixture is stirred for one additional hour at 23°C. Thereupon the
chloroform solution is diluted with 200 parts by weight of ether and agitated
with 3 N hydrochloric acid. The weakly Congo acid solution is heated for 1
hour in a steam bath, the acetyl group of the reaction product being thereby
split off, and the mixture is filtered over charcoal.
Upon adding concentrated ammonia in excess, the condensation product
separates and is taken up in chloroform. The chloroform solution is dried and
distilled, the tropic acid N-ethyl-N-(γ-picolyl)-amide being thereby obtained in
the form of a thick oil, which crystallizes after prolonged time and which then
melts at 96° to 97°C. | | brand name | Mydriacyl (Alcon); Tropicacyl (Akorn). | | Therapeutic Function | Anticholinergic (ophthalmic) | | 一般的な説明 | Tropicamide, N-ethyl-2-phenyl-N-(4-pyridylmethyl)hydracrylamide (Mydriacyl), is aneffective anticholinergic for ophthalmic use when mydriasisis produced by relaxation of the sphincter muscle ofthe iris, allowing adrenergic innervation of the radial muscleto dilate the pupil. Its maximum effect is achieved inabout 20 to 25 minutes and lasts for about 20 minutes,with complete recovery in about 6 hours. Its action ismore rapid in onset and wears off more rapidly thanthat of most other mydriatics. To achieve mydriasis, either0.5% or 1.0% concentration may be used, althoughcycloplegia is achieved only with the stronger solution.Its uses are much the same as those described above formydriatics in general, but opinions differ on whether thedrug is as effective as homatropine, for example, inachieving cycloplegia. For mydriatic use, however, in examinationof the fundus and treatment of acute iritis,iridocyclitis, and keratitis, it is quite adequate; and becauseof its shorter duration of action, it is less prone toinitiate a rise in intraocular pressure than the more potent,longer-lasting drugs. As with other mydriatics, however,pupil dilation can lead to increased intraocular pressure.In common with other mydriatics, it is contraindicated inpatients with glaucoma, either known or suspected, andshould not be used in the presence of a shallow anteriorchamber. Thus far, no allergic reactions or ocular damagehas been observed with this drug. The ability to clone thevarious muscarinic receptor subtypes has allowed the observationthat tropicamide has modest selectivity for theM4 receptor. | | 生物活性 | M 4 selective muscarinic receptor antagonist. | | Biochem/physiol Actions | M4 muscarinic acetylcholine receptor antagonist. | | 合成 | Tropicamide, N-(4-piridinylmethyl)-N-ethyl-|?-hydroxy-|á-phenylpropi�onamide (14.1.41), is synthesized by reacting O-acetyltropyl chloride with ethyl
(4-piridinylmethyl)amine and the subsequent acidic hydrolysis of the acetyl group in the
resulting amide (14.1.40) [31]. 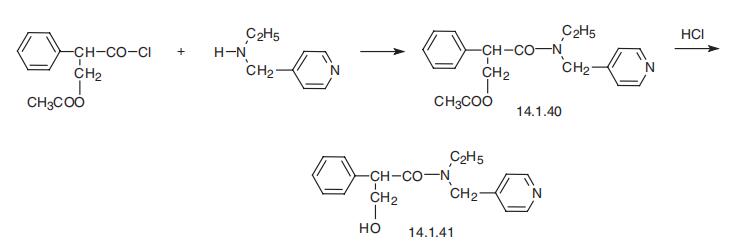
| | Veterinary Drugs and Treatments | Tropicamide, like atropine, causes mydriasis and cycloplegia, but
has more mydriatic than cycloplegic activity. Tropicamide has a
more rapid onset (maximum mydriasis in 15 – 30 minutes) of action
and a shorter duration of action (pupil returns to normal in
6 – 12 hours in most animals) than does atropine, thereby making
it more useful for funduscopic examinations. In dogs, intraocular
pressure is apparently not affected by tropicamide. Tropicamide is
also indicated following cataract removal to prevent synechiae formation
that is associated with post-cataract atropine administration.
As the half-life of tropicamide is shorter than that of atropine,
this allows iris contraction preventing synechial adhesions. | | 貯蔵 | Store at RT |
|