|
ChemicalBook Optimization Suppliers |
|
| | ビス(トリフェニルホスフィン)パラジウム(II)ジクロリド 製品概要 |
| | ビス(トリフェニルホスフィン)パラジウム(II)ジクロリド 物理性質 |
| 融点 | 260°C | | 蒸気圧 | 0Pa at 25℃ | | RTECS 番号 | RT3578000 | | 貯蔵温度 | Store below +30°C. | | 溶解性 | Chloroform (Slightly), Dichloromethane (Slightly, Heated), Methanol (Slightly, | | 外見 | Powder | | 色 | Yellow | | 水溶解度 | Insoluble in water. Soluble in benzene, and toluene. | | Sensitive | Hygroscopic | | BRN | 4935975 | | InChI | InChI=1S/2C18H15P.2ClH.Pd/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2 | | InChIKey | ILBDOZRDKNIJBS-UHFFFAOYSA-N | | SMILES | P(C1C=CC=CC=1)(C1=CC=CC=C1)C1C=CC=CC=1.P(C1C=CC=CC=1)(C1C=CC=CC=1)C1C=CC=CC=1.[Pd](Cl)Cl | | LogP | 5.69 at 20℃ | | CAS データベース | 13965-03-2(CAS DataBase Reference) |
| | ビス(トリフェニルホスフィン)パラジウム(II)ジクロリド Usage And Synthesis |
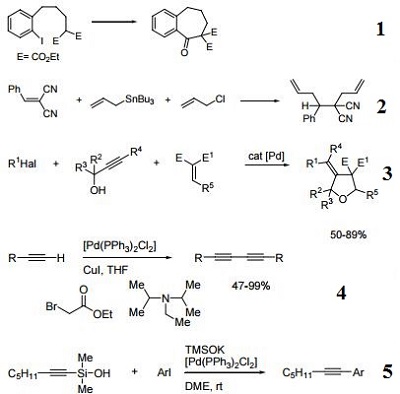
| 外観 | 黄色〜黄赤色, 結晶性粉末〜粉末又は塊 | | 溶解性 | 水、エタノール及びアセトンに難溶、N,N-ジメチルホルムアミドに溶ける。 | | 用途 | 薗頭カップリングなどの触媒に使われている。 | | 用途 | 有機合成用均質触媒。 | | 使用上の注意 | 不活性ガス封入 | | 化学的特性 | Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. This yellow solid is insoluble in water but can dissolve in some organic solvents, such as toluene and benzene, and is slightly soluble in acetone and chloroform. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex adopts a square planar geometry and numerous similar complexes are known, with different phosphine ligands. | | 使用 | Bis(triphenylphosphine)palladium(II) chloride is used primarily in organometallic catalytic reactions due to the palladium content of the compound. It is also involved in crystalized structures of me tallacyclic complexes which show antiinflammatory and antifungal properties. | | 合成 | The synthesis of Bis(triphenylphosphine)palladium(II) chloride can be achieved by the reaction of palladium(II) chloride with triphenylphosphine, resulting in the formation of Bis(triphenylphosphine)palladium(II) chloride. This chemical process is represented by the balanced equation: PdCl2 + 2 PPh3 → PdCl2(PPh3)2. | | 反応性 | Precatalyst for the carbonylative cyclization of malonate derivatives.
Catalyst used in the double allylation of activated olefins.
Precatalyst for the three-component preparation of 3-arylidene- (or 3-alkenylidene) tetrahydrofurans.
Precatalyst for the homocoupling of terminal alkynes.
Precatalyst in the cross-coupling of alkynylsilanols and aryl halides.
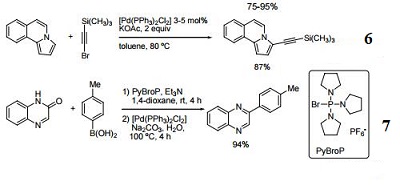
Catalyst for direct Pd-catalyzed alkynylation of N-fused heterocycles.
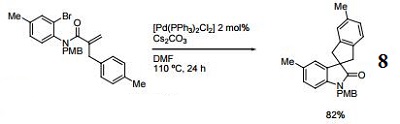
Catalyst for a tandem Heck reaction/C-H functionalization.
Catalyst for direct arylation of tautomerizable heterocycles.
 | | 一般的な説明 | Bis(triphenylphosphine)palladium(II) dichloride is an organometallic complex. It is an efficient cross-coupling catalyst for C-C coupling reaction, such as Negishi coupling, Suzuki coupling, Sonogashira coupling and Heck coupling reaction. Detection of bis(triphenylphosphine)palladium(II) dichloride by electrospray ionization quadrupole ion trap mass spectrometry using different imidazolium salts as the charge carrier has been reported. It is employed as catalyst for the Heck reaction medium. | | 燃焼性と爆発性 | Non flammable |
|