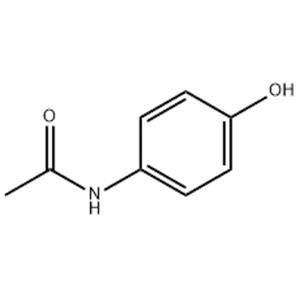
Acetaminophen NEW
| Price | $6 | $5 | $1 |
| Package | 1KG | 25KG | 100KG |
| Min. Order: | 1KG |
| Supply Ability: | g-kg-tons, free sample is available |
| Update Time: | 2024-03-29 |
Product Details
| Product Name: Acetaminophen | CAS No.: 103-90-2 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: g-kg-tons, free sample is available | Release date: 2024/03/29 |
| Lead time: In stock | Packaging: bottle/drum/bucket/IBC, as request. |
| Delivery: By sea, by air, by express | Origin: Manufacturer |
| Name: Sun |
1. Product information
| Name | paracetamol |
|---|---|
| Synonym | More Synonyms |
| Description | Acetaminophen (paracetamol) is a selective cyclooxygenase-2 (COX-2) inhibitor with an IC50 of 25.8 μM; is a widely used antipyretic and analgesic drug. |
|---|---|
| Related Catalog | Signaling Pathways >> Immunology/Inflammation >> COX Research Areas >> Inflammation/Immunology Natural Products >> Phenylpropanoids |
| Target | COX-2:25.8 μM (IC50) COX-1:113.7 μM (IC50) Human Endogenous Metabolite |
| In Vitro | In vitro, acetaminophen elicites a 4.4-fold selectivity toward COX-2 inhibition (IC50 113.7 μM for COX-1; IC50 25.8 μM for COX-2). Following oral administration of the drug, maximal ex vivo inhibitions are 56% (COX-1) and 83% (COX-2). Acetaminophen plasma concentrations remaine above the in vitro IC50 for COX-2 for at least 5 h postadministration. Ex vivo IC50 values (COX-1: 105.2 μM; COX-2: 26.3 μM) of acetaminophen compared favorably with its in vitro IC50 values. In contrast to previous concepts, acetaminophen inhibited COX-2 by more than 80%, i.e., to a degree comparable to nonsteroidal antiinflammatory drugs (NSAIDs) and selective COX-2 inhibitors. However, a >95% COX-1 blockade relevant for suppression of platelet function is not achieved[1]. MTT assay shows that Acetaminophen (APAP) in a dose of 50 mM significantly (p<0.001) reduces cell viability to 61.5±6.65%. Interestingly, the significant (p<0.01) increase in cell viability to 79.7±2.47% is observed in the Acetaminophen/HV110 co-treated cells, compared to Acetaminophen treated cells[2]. |
| In Vivo | Administering Acetaminophen (250 mg/kg, orally) to the mice causes significant (p<0.001) liver damage and necrosis of cells as evidenced by the elevated serum hepatic enzymes alanine aminotransferase (ALT), aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyl transferase (γGT) compared with normal group. Conversely, effects of pretreatment with different doses of citral (125, 250, and 500 mg/kg) exhibited a significant (p<0.05) decrease in serum activities of ALT (91.79%, 93.07%, and 95.61%, resp.), AST (93.40%, 91.89%, and 96.52%, resp.), ALP (39.29%, 37.07%, and 59.80%, resp.), and γGT (92.83%, 91.59%, and 93.0%, resp.), when compared to the Acetaminophen group. Similar results were found in pretreatment with SLM on the activity of ALT (95.90%), AST (95.03%), ALP (70.52%), and γGT (92.69%)[3]. |
| Cell Assay | Human hepatoma cell line HepG2 is cultured in low glucose DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/mL Penicillin and 100 μg/mL Streptomycin and 2 mM l-glutamine. The cells are maintained in 75 cm2 flasks at 37°C in a humidified atmosphere containing 5% CO2 and split at 80% confluence every 5 days. Cells are seeded in 24-well plate (2×105 cells) and incubated at 37°C overnight followed by cells pretreatment with complete DMEM containing high glucose concentration in order to downregulate autophagy. After 6 h, cells are treated with different concentrations of postbiotics obtained from Lactobacillus fermentum BGHV110 strain (HV110) in order to select appropriate dose for further experiments. Postbiotic is dissolved in complete DMEM medium and added to the cells in specific final concentration. In all other experiments seeded cells are treated with 50 mM Acetaminophen alone or co-treated with 50 mM Acetaminophen and selected dose of lyophilized HV110. To analyze autophagic flux, simultaneously with treatments, cells are exposed to lysosomotropic agent Chloroquine at a concentration of 25 μM, to inhibit autophagosome-lysosome fusion[2]. |
| Animal Admin | Mice[3] Male Swiss mice (30-40 g) are used. The experimental animals are divided into six groups of five animals each. Firstly, each group receive orally during seven days the following treatment: Group I: the mice do not receive any treatment (normal). Group II: the mice receive citral vehicle (0.1% Tween 80 solution). Groups III-V: the mice are pretreated with citral at doses of 125, 250, and 500 mg/kg, respectively. Group VI: the mice are pretreated with the hepatoprotective standard drug Silymarin (SLM) (200 mg/kg). After this time, the animals fasted for 8 h and then receive oral Acetaminophen on the seventh day at a dose of 250 mg/kg in Groups II-VI. Group I orally receive saline that contained 0.1% Tween 80 solution (Acetaminophen vehicle). The stock solution is used as the first concentration of 50 mg/mL and after that is diluted in 0.1% Tween 80 solution to prepare the solutions of 25 and 12.5 mg/mL. After 12 h of Acetaminophen administration, serum samples and liver tissue are collected followed by biochemistry and histological analysis. |
| References | [1]. Hinz, B, et al. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J, 2008. 22(2): p. 383-90. [2]. Dini? M, et al. Lactobacillus fermentum Postbiotic-induced Autophagy as Potential Approach for Treatment ofAcetaminophen Hepatotoxicity. Front Microbiol. 2017 Apr 6;8:594. [3]. Uchida NS, et al. Hepatoprotective Effect of Citral on Acetaminophen-Induced Liver Toxicity in Mice. Evid Based Complement Alternat Med. 2017;2017:1796209. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 387.8±25.0 °C at 760 mmHg |
| Melting Point | 168-172 °C(lit.) |
| Molecular Formula | C8H9NO2 |
| Molecular Weight | 151.163 |
| Flash Point | 188.4±23.2 °C |
| Exact Mass | 151.063324 |
| PSA | 49.33000 |
| LogP | 0.34 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.619 |
| Storage condition | Store at RT |
| Water Solubility | 14 g/L (20 ºC) |
4-Acetamidophenol MSDS(Chinese) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |  GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H317-H319 |
| Precautionary Statements | P280-P301 + P312 + P330-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22:Harmful if swallowed. R36/37/38:Irritating to eyes, respiratory system and skin . R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment . R36/38:Irritating to eyes and skin . R40:Limited evidence of a carci |
| Safety Phrases | S26-S36-S61-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | AE4200000 |
| HS Code | 29242930 |
| Precursor 0 | |
|---|---|
| DownStream 10 | Previous 1/3 Next |
| |
| HS Code | 29242930 |
|---|
| Characterization of a highly sensitive and selective novel trapping reagent, stable isotope labeled glutathione ethyl ester, for the detection of reactive metabolites. J. Pharmacol. Toxicol. Methods 76 , 83-95, (2015) Glutathione (GSH) trapping assays are widely used to predict the post-marketing risk for idiosyncratic drug reactions (IDRs) in the pharmaceutical industry. Although several GSH derivatives have been ... | |
| Application of IL-36 receptor antagonist weakens CCL20 expression and impairs recovery in the late phase of murine acetaminophen-induced liver injury. Sci. Rep. 5 , 8521, (2015) Overdosing of the analgesic acetaminophen (APAP, paracetamol) is a major cause of acute liver injury. Whereas toxicity is initiated by hepatocyte necrosis, course of disease is regulated by mechanisms... | |
| Simple and reliable HPLC method for the monitoring of methotrexate in osteosarcoma patients. J. Chromatogr. Sci. 52(7) , 590-5, (2014) Methotrexate (MTX) is a dihydrofolate reductase inhibitor that is used for the treatment of tumors and autoimmune diseases. Several automated binding assays are used in clinical practice and numerous ... |
| APAP |
| Calpol |
| NAPAP |
| p-acetaminophenol |
| Panadol |
| Tylenol |
| Exdol |
| Acetamide, N-(4-hydroxyphenyl)- |
| 4-(Acetylamino)phenol |
| Acetamide, N-(p-hydroxyphenyl)- |
| Fevor |
| Acetanilide, 4'-hydroxy- |
| N-(p-hydroxyphenyl)acetamide |
| Paracetamol |
| N-(4-Hydroxy-phenyl)-acetamide |
| Korum |
| 4-13-00-01091 (Beilstein Handbook Reference) |
| p-hydroxyacetanilide |
| G 1 |
| p-Acetylaminophenol |
| MFCD00002328 |
| Panex |
| QR DMV1 |
| N-(4-Hydroxyphenyl)acetamide |
| N-Acetyl-4-aminophenol |
| EINECS 203-157-5 |
| 4'-Hydroxyacetanilide |
| N-(4-Hydroxyphenyl)acetanilide |
| Acetaminophen |
| p-(Acetylamino)phenol |
| Phenol, p-acetamido- |
| 4-Acetaminophenol |
| Dirox |
| 4-Acetamidophenol |
| Dypap |
| Hedex |
| N-Acetyl-p-aminophenol |
2. Packaging
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping

4. Contact information
For more details, pls contact us freely.
Email address : Sun@fdachem.com
Mob: 86 13526505137
WhatsApp/Skype/Wechat/LINE: 86 13526505137
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $15.00/1kg |
VIP1Y
|
Ouhuang Engineering Materials (Hubei) Co., Ltd
|
2024-04-28 | |
| $0.00/1kg |
VIP1Y
|
Henan Suikang Pharmaceutical Co.,Ltd.
|
2024-04-26 | |
| $0.00/1kg |
VIP1Y
|
Shanghai Affida new material science and technology center
|
2024-04-17 | |
| $10.00/1kg |
VIP1Y
|
Hunan aslsen technology co.,ltd
|
2024-04-10 | |
| $0.00/1kg |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-09 | |
| $25.00/1kg |
Wuhan Boyuan Import & Export Co., LTD
|
2024-04-08 | ||
| $3.00/1kg |
VIP1Y
|
Shanghai Aosiris new Material Technology Co., LTD
|
2024-04-01 | |
| $20.00/1kg |
VIP1Y
|
Shaanxi Franta Biotechnology Co., Ltd
|
2024-03-27 | |
| $6.00/1KG |
VIP1Y
|
Hebei Saisier Technology Co., LTD
|
2024-03-26 | |
| $1.00/1kg |
VIP4Y
|
Dorne Chemical Technology co. LTD
|
2024-03-26 |
- Since: 2023-02-10
- Address: Room 01, 2288 E05, Building 14, East Henan University, Science and Technology Park, 279 Xisanhuan Ro



 CAS#:105326-63-4
CAS#:105326-63-4 CAS#:109690-44-0
CAS#:109690-44-0 CAS#:30824-21-6
CAS#:30824-21-6 CAS#:3273-78-7
CAS#:3273-78-7

 China
China