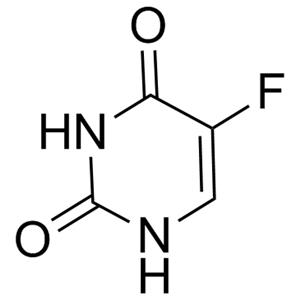
Product Details
| Product Name: 5-Fluorouracil | CAS No.: 51-21-8 |
| Min. Order: 1KG | Purity: 98% |
| Supply Ability: g-kg-tons, free sample is available | Release date: 2025/09/25 |
| Lead time: In stock, ready for shipment | Packaging: bag/bottle/drum/IBC |
| Delivery: By express, by air, by sea | Delivery: Manufacturer, advantage product |
| COA, MSDS: Manufacturer, advantage product | Name: Tina |
1. Materials information
| Name | 5-fluorouracil |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Fluorouracil is a potent antitumor agent that affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. |
|---|---|
| Related Catalog | Signaling Pathways >> Cell Cycle/DNA Damage >> Nucleoside Antimetabolite/Analog Research Areas >> Cancer |
| In Vitro | 5-Fluorouracil (5-Fu) and doxorubicin (Dox) show synergistic anticancer efficacy. The IC50 value of 5-Fu/Dox-DNM toward human breast cancer (MDA-MB-231) cells is 0.25 μg/mL, presenting an 11.2-fold and 6.1-fold increase in cytotoxicity compared to Dox-DNM and 5-Fu-DNM, respectively[1]. In 5-fluorouracil (5-FU) and CDDP treated NFBD1-inhibited NPC cells, the NFBD1 expression in NPC CNE1 cell lines is depleted using lentivirus-mediated short hairpin RNA, and the sensitivity of these cells is elevated. NFBD1 knockdown leads to an obvious induction of apoptosis in CDDP- or 5-FU-treated CNE1 cells[3]. |
| In Vivo | 5-Fluorouracil (23 mg/kg, 3 times/week) for 14 days, induces accelerated gastrointestinal transit associated with acute intestinal inflammation at day 3 after the start of treatment, which may have led to persistent changes in the ENS observed after days 7 and 14 of treatment contributing to delayed gastrointestinal transit and colonic dysmotility[2]. |
| Animal Admin | Mice receive intraperitoneal injections of 5-FU (23 mg/kg), 3 times a week via a 26 gauge needle. 5-FU is dissolved in 100% dimethyl sulfoxide (DMSO) to make 1 M/L stock solution refrigerated at −20°C. The stock is then defrosted and diluted with sterile water to make 0.1 M/L (10% DMSO) solutions for intraperitoneal injections. The dose of 5-FU is calculated to be equivalent to standard human dose per body surface area. The low doses of 5-FU (10-40 mg/kg) have been shown to have antitumor efficacy in mouse models of cancer. Sham-treated mice received 10% DMSO in sterile water via intraperitoneal injection three times a week via a 26 gauge needle. The injected volumes are calculated to the body weight; the maximum volume does not exceed 200 μL per injection. Mice are euthanized via cervical dislocation at 3 (2 treatments), 7 (3 treatments), and 14 (6 treatments) days after the first injection and colon is collected for in vitro experiments. |
| References | [1]. Han R, et al. Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy. J Drug Target. 2016 Jun 29:1-28. [Epub ahead of print] [2]. McQuade RM, et al. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol Motil. 2016 Jun 28. [3]. Zeng Q, et al. Knockdown of NFBD1/MDC1 enhances chemosensitivity to cisplatin or 5-fluorouracil in nasopharyngeal carcinoma CNE1 cells. Mol Cell Biochem. 2016 Jul;418(1-2):137-46. [4]. Yin L, et al. Antitumor effects of oncolytic herpes simplex virus type 2 against colorectal cancer in vitro and in vivo. Ther Clin Risk Manag. 2017 Feb 7;13:117-130. |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 401.4±48.0 °C at 760 mmHg |
| Melting Point | 282-286 °C (dec.)(lit.) |
| Molecular Formula | C4H3FN2O2 |
| Molecular Weight | 130.077 |
| Flash Point | 196.5±29.6 °C |
| Exact Mass | 130.017853 |
| PSA | 65.72000 |
| LogP | -2.10 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.596 |
| Storage condition | Store at 0-5 |
| Stability | Stable. Light sensitive. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| Water Solubility | 12.2 g/L 20 ºC |
Fluorouracil MSDS(Chinese) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |  GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H412 |
| Precautionary Statements | P273-P301 + P310 |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36-S36/37 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | YR0350000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2933599090 |
 cytosine 71-30-7 ~79% 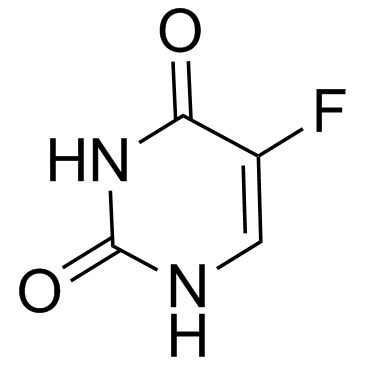 Fluorouracil 51-21-8 |
| Literature: Daikin Kogyo Co., Ltd.; Asahi Chemical Industry Co., Ltd. Patent: US4122251 A1, 1978 ; |
 Uracil 66-22-8 ~92%  Fluorouracil 51-21-8 |
| Literature: Yemul, S. S.; Kagan, H. B.; Setton, R. Tetrahedron Letters, 1980 , vol. 21, p. 277 - 280 |
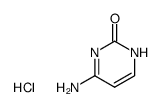 Cytosine hydroc... 1784-08-3 ~64%  Fluorouracil 51-21-8 |
| Literature: Daikin Kogyo Co., Ltd.; Asahi Chemical Industry Co., Ltd. Patent: US4122251 A1, 1978 ; |
| Precursor 9 | Previous 1/3 Next |
|---|---|
| |
| DownStream 10 | Previous 1/3 Next |
| |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
2. Packaging of materials
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping & Delivery
By Express
Provide door to door service
Suitable for goods under 50kg
Delivery: 3-7 days
Cost: low cost

By Air
Provide airport to airport service
Suitable for goods over 50kg
Delivery: 3-14 days
Cost: high cost

By Sea
Provide seaport to seaport service
Suitable for goods over 100kg
Delivery: 2-45 days
Cost: low cost

4. Contact information
For more details, pls contact us freely.
Email address: Tina@fdachem.com
Mob: 86 13213167925
WhatsApp/Skype/Wechat/LINE: 86 13213167925
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $48.00/1g |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-12-03 | |
| $48.00/1g |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-12-03 | |
| $0.00/1kg |
VIP2Y
|
HANGZHOU HAILAN CHEMICAL CO.,LTD.
|
2025-08-26 | |
| $5.00/1KG |
VIP1Y
|
Wuhan JiyunZen Tech Co., Ltd.
|
2025-06-07 | |
| $/ |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-04-29 | |
| $48.00/1g |
VIP2Y
|
TargetMol Chemicals Inc.
|
2025-04-27 | |
| $25.00/1ASSAYS |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-18 | |
| $0.00/5Kg |
VIP2Y
|
Xinxiang Aurora Biotechnology Co.,Ltd.
|
2024-11-13 | |
| $100.00/1kg |
VIP5Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-22 | |
| $60.00/1kg |
VIP2Y
|
S&Y Biochem Co.,Ltd
|
2024-08-01 |


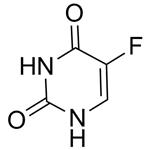


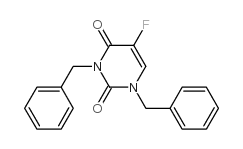 CAS#:75500-02-6
CAS#:75500-02-6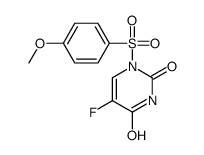 CAS#:105434-88-6
CAS#:105434-88-6 CAS#:105411-89-0
CAS#:105411-89-0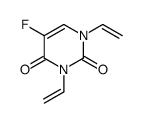 CAS#:111854-27-4
CAS#:111854-27-4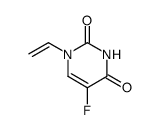 CAS#:72975-39-4
CAS#:72975-39-4![(5''-bromo-3,3''-dioctyl-[2,2':5',2''-terthiophene]-5-carbaldehyde](https://img.chemicalbook.com/ProductImageEN/2023-12/Large/943f5d11-92f9-4154-876f-1bb4eb5ff139.gif)

 China
China