
Sorafenib tosylate
| Price | $7 |
| Package | 1KG |
| Min. Order: | 1KG |
| Supply Ability: | JD 439 |
| Update Time: | 2019-09-02 |
Product Details
| Product Name: Sorafenib tosylate | CAS No.: 284461-73-0 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: JD 439 | Release date: 2019/09/02 |
▼
▲
Product Name:
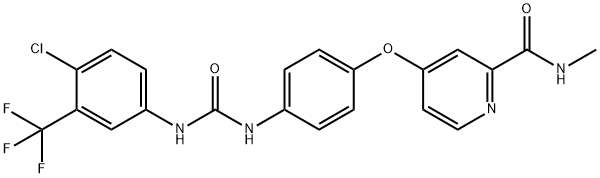
Sorafenib tosylate
Synonyms:
Sorafenib free base for research;Sorafenib Free Base;Forafenib;Sorafenib free base(Bay43-9006);nexavar,sorafenib;Nexavar;Sorafenib-d4;N-[4-Chloro-3-(trifluoromethyl)phenyl]-({4-[2-(N-methyl-carbamoyl)(4-pyridyloxy)]phenyl}amino)-carboxamide
CAS:
284461-73-0
MF:
C21H16ClF3N4O3
MW:
464.83
EINECS:
608-209-4
Product Categories:
anti-neoplastic;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Pharmaceutical intermediate;Amines;-;Inhibitor;Sorafinib;Molecular Targeted Antineoplastic;All Inhibitors;Bay 43-9006
Mol File:
284461-73-0.mol
▼
▲
Sorafenib tosylate Chemical Properties
▼
▲
Melting point
202-204°C
storage temp.
-20°C Freezer
form
White to off-white solid.
CAS DataBase Reference
284461-73-0(CAS DataBase Reference)
▼
▲
Safety Information
▼
▲
Risk Statements
68/20/21/22-37/38
Safety Statements
36-37-39
HS Code
29350090
▼
▲
MSDS Information
▼
▲
Sorafenib tosylate Usage And Synthesis
▼
▲
Overview
Sorafenib tosylate is the tosylate form of sorafenib, which is a drug approved for the treatment of hepatocellular carcinoma and the treatment of advanced renal cell carcinoma (primary kidney cancer). Hepatocellular carcinoma accounts for the vast majority of primary liver cancers (85–90%). [1] Approximately 70–90% of all hepatocellular cancer cases occur in patients with chronic liver disease and cirrhosis, with the main causes of cirrhosis including hepatitis B, hepatitis C and alcoholic liver disease.[1] Sorafenib is an oral receptor tyrosine kinase inhibitor that inhibits Raf serine/threonine kinases and receptor tyrosine kinases (vascular endothelial growth factor receptors 1, 2, 3 and platelet-derived growth factor-b, Flt-3 and c-kit) that are implicated in tumorigenesis and tumor progression.
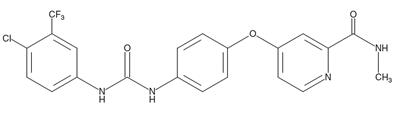
Figure 1 the chemical structure of sorafenib;

Figure 1 the chemical structure of sorafenib;
Indications
It is indicated for the treatment of hepatocellular carcinoma and the treatment of advanced renal cell carcinoma (primary kidney cancer).
Mechanism of action
The bi-aryl urea sorafenib is an oral multikinase inhibitor that inhibits both cell surface tyrosine kinase receptors and downstream intracellular serine/threonine kinases in the Ras/MAPK cascade.[2-4] Receptor tyrosine kinases inhibited by sorafenib include vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)-b, c-KIT, FMS-like tyrosine kinase 3 (FLT-3) and RET. Intracellular Raf serine/threonine kinase isoforms inhibited by sorafenib include Raf-1 (or C-Raf), wild-type B-Raf and mutant B-Raf.[3, 4] These kinases are involved in tumour cell proliferation and tumour angiogenesis.[3, 4]
The antiproliferative activity of sorafenib is variable in different tumor types and largely depends on the oncogenic signaling pathways that mediate tumor proliferation. Sorafenib has also been shown to induce apoptosis in several tumor cell lines. Although the mechanism through which sorafenib induces apoptosis is not fully elucidated and may vary between cell lines, a commonly observed theme is the inhibition of phosphorylation of the initiation factor eIF4E and loss of the antiapoptotic protein myeloid cell leukemia-1 (MCL-1)[5]. Recently, sorafenib was shown to inhibit hepatitis C viral replication in vitro[6], and in vitro studies have also shown some direct effects on immune cells [7]. Whether these effects
The antiproliferative activity of sorafenib is variable in different tumor types and largely depends on the oncogenic signaling pathways that mediate tumor proliferation. Sorafenib has also been shown to induce apoptosis in several tumor cell lines. Although the mechanism through which sorafenib induces apoptosis is not fully elucidated and may vary between cell lines, a commonly observed theme is the inhibition of phosphorylation of the initiation factor eIF4E and loss of the antiapoptotic protein myeloid cell leukemia-1 (MCL-1)[5]. Recently, sorafenib was shown to inhibit hepatitis C viral replication in vitro[6], and in vitro studies have also shown some direct effects on immune cells [7]. Whether these effects
Side effects
The most common adverse reactions (20%), considered to be related to sorafenib, in patients with HCC or RCC are fatigue, weight loss, rash/desquamation, hand-foot skin reaction, alopecia, diarrhea, anorexia, nausea and abdominal pain [12].
Across all tumor types, common side effects (> 10%) include hypertension (9 -13%, grade 4: < 1%; onset: ~ 3 weeks), fatigue (37 -46%), sensory neuropathy (13%), pain (11%), rash/desquamation (19 -40%; grade 3: 1%), handfoot syndrome (21 -30%; grade 3: 6 -8%), alopecia (14 -27%), pruritis (14 -19%), dry skin (10 -11%), hypoalbuminemia (59%), hypophosphatemia (35 -45%; grade 3: 11 -13%; grade 4: < 1%), diarrhea (43 -55%; grade 3: 2 -10%; grade 4: < 1%), lipase increased (40 -41%, usually transient), amylase increased (30 -34, usually transient), abdominal pain (11 -31%), weight loss (10 -30%), anorexia (16 -29%), nausea (23 -24%), vomiting (15 -16%), constipation (14 -15%), muscle pain, weakness, dyspnea (14%), cough (13%) and hemorrhage (15 -18%; grade 3: 2 -3%; grade 4: 2%). Laboratory abnormalities attributable to sorafenib use are also seen and include lymphopenia (23 -47%; grades 3/4: 13%), thrombocytopenia (12 -46%; grades 3/4: 1 -4%), international normalized ration (INR) increased (42%), neutropenia (18%; grades 3/4: 5%), leucopenia, liver dysfunction (11%; grade 3: 2%; grade 4: 1%).
Less frequent side effects (> 1 -10) include cardiac ischemia/infarction (3%), flushing, headache (10%), depression, fever, acne, exfoliative dermatitis, decreased appetite, dyspepsia, dysphagia, esophageal varices bleeding (2%), glossodynia, mucositis, stomatitis, xerostomia, erectile dysfunction, anemia, transaminases increased (transient), joint pain (10%), arthralgia, myalgia, hoarseness and flu-like syndrome.
Rare (< 1%) side effects of sorafenib include acute renal failure, alkaline phosphatase increased, arrhythmia, bilirubin increased, bone pain, cardiac failure, cerebral hemorrhage, congestive heart failure, dehydration, eczema, epistaxis, erythema multiforme, folliculitis, gastritis, gastrointestinal hemorrhage, gastrointestinal perforation, gastrointestinal reflux, gynecomastia, hypersensitivity (skin reaction, urticaria), hypertensive crisis, hyponatremia, hypothyroidism, infection, jaundice, myocardial infarction (MI), mouth pain, myocardial ischemia, pancreatitis, pleural effusion, preeclampsialike syndrome (reversible hypertension and proteinuria), renal failure, respiratory hemorrhage, reversible posterior leukoencephalopathy syndrome (RPLS), rhinorrhea, skin cancer (squamous cell/keratoacanthomas), thromboembolism, tinnitus, transient ischemic attack, tumor lysis syndrome, tumor pain and voice alteration.
Across all tumor types, common side effects (> 10%) include hypertension (9 -13%, grade 4: < 1%; onset: ~ 3 weeks), fatigue (37 -46%), sensory neuropathy (13%), pain (11%), rash/desquamation (19 -40%; grade 3: 1%), handfoot syndrome (21 -30%; grade 3: 6 -8%), alopecia (14 -27%), pruritis (14 -19%), dry skin (10 -11%), hypoalbuminemia (59%), hypophosphatemia (35 -45%; grade 3: 11 -13%; grade 4: < 1%), diarrhea (43 -55%; grade 3: 2 -10%; grade 4: < 1%), lipase increased (40 -41%, usually transient), amylase increased (30 -34, usually transient), abdominal pain (11 -31%), weight loss (10 -30%), anorexia (16 -29%), nausea (23 -24%), vomiting (15 -16%), constipation (14 -15%), muscle pain, weakness, dyspnea (14%), cough (13%) and hemorrhage (15 -18%; grade 3: 2 -3%; grade 4: 2%). Laboratory abnormalities attributable to sorafenib use are also seen and include lymphopenia (23 -47%; grades 3/4: 13%), thrombocytopenia (12 -46%; grades 3/4: 1 -4%), international normalized ration (INR) increased (42%), neutropenia (18%; grades 3/4: 5%), leucopenia, liver dysfunction (11%; grade 3: 2%; grade 4: 1%).
Less frequent side effects (> 1 -10) include cardiac ischemia/infarction (3%), flushing, headache (10%), depression, fever, acne, exfoliative dermatitis, decreased appetite, dyspepsia, dysphagia, esophageal varices bleeding (2%), glossodynia, mucositis, stomatitis, xerostomia, erectile dysfunction, anemia, transaminases increased (transient), joint pain (10%), arthralgia, myalgia, hoarseness and flu-like syndrome.
Rare (< 1%) side effects of sorafenib include acute renal failure, alkaline phosphatase increased, arrhythmia, bilirubin increased, bone pain, cardiac failure, cerebral hemorrhage, congestive heart failure, dehydration, eczema, epistaxis, erythema multiforme, folliculitis, gastritis, gastrointestinal hemorrhage, gastrointestinal perforation, gastrointestinal reflux, gynecomastia, hypersensitivity (skin reaction, urticaria), hypertensive crisis, hyponatremia, hypothyroidism, infection, jaundice, myocardial infarction (MI), mouth pain, myocardial ischemia, pancreatitis, pleural effusion, preeclampsialike syndrome (reversible hypertension and proteinuria), renal failure, respiratory hemorrhage, reversible posterior leukoencephalopathy syndrome (RPLS), rhinorrhea, skin cancer (squamous cell/keratoacanthomas), thromboembolism, tinnitus, transient ischemic attack, tumor lysis syndrome, tumor pain and voice alteration.
References
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007 Jun; 132 (7): 2557-76
- Adnane L, Trail PA, Taylor I, et al. Sorafenib (BAY 439006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol 2005; 407: 597-612
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006 Oct; 5 (10): 835-44
- Wilhelm SM, Carter C, Tang LY, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004 Oct 1; 64 (19): 7099-109
- Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 2005;24:6861-9
- Himmelsbach K, Sauter D, Baumert TF, et al. New aspects of an anti-tumour drug: sorafenib efficiently inhibits HCV replication. Gut 2009;58:1644-53
- Molhoek KR, McSkimming CC, Olson WC, et al. Apoptosis of CD4(+) CD25(high) T cells in response to Sirolimus requires activation of T cell receptor and is modulated by IL-2. Cancer Immunol Immunother 2009;58:867-76
- Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965-72
- Clinical Pharmacology and Biopharmaceutics NDA Review for Sorafenib Tosylate (NDA 21 923), F.C.F.D.E.A. RESEARCH, Editor, 2005
- BAY 43-9006 (sorafenib) Investigator’s Brochure. Bayer Healthcare AG,Version 10.0, July 1, 2009
- European Medicines Agency. Sorafenib (Nexavar): summary of product characteristics [online].
- Blanchet B, Billemont B, Barete S, et al. Toxicity of sorafenib: clinical and molecular aspects. Expert Opin Drug Saf 2010;9:275-87
- https://www.drugs.com/cdi/sorafenib.html
Description
Sorafenib is a small molecular inhibitor of several kinases involved in tumor angiogenesis and proliferation, including, but not limited to, Raf (IC50=12nM for Raf-1), VEGFR (IC50=90nM for VEGFR-2 and IC50=12nM for VEGFR-3), and platelet derived growth factor receptor (IC50=57nM for PDGFR-b). Specifically, sorafenib blocks tumor progression by inhibiting cellular proliferation that is dependent on activation of the MAPK pathway (Raf) and/or inhibiting tumor angiogenesis through VEGFR and/or PDGFR. While it may be effective in the treatment of a variety of tumors, the first approvable indication is for renal cell carcinoma. Overall, the drug appears to be well tolerated by the majority of patients at the 400 mg b.i.d. continuous dosing. As an inhibitor of multiple kinases vital for tumor progression, sorafenib may possess wide-spectrum antitumor properties and may emerge as an effective weapon against a variety of solid tumors.
Chemical Properties
Light Yellow Solid
Originator
Bayer/Onyx (Germany)
Uses
A potent RAF kinase inhibitor. Antineoplastic
Uses
Multiple kinase inhibitor targeting both RAF kinase and receptor tyrosine kinases that promote angiogensis. Antineoplastic.
Uses
Sorafenib Tosylate (Bay 43-9006, Nexavar) is a small molecular inhibitor of VEGFR, PDGFR, c-Raf and B-Raf with IC50s of 18 nM, 10 nM, 3 nM and 15 nM, respectively.
Uses
Sorafenib Tosylate (Bay 43-9006) is a multikinase inhibitor of Raf-1, B-Raf and VEGFR-2 with IC50 of 6 nM, 22 nM and 90 nM, respectively - See more at: http://www.selleckchem.com/products/Sorafenib-Tosylate.html#sthash.BjHEmCf3.dpuf
Indications
Sorafenib (Nexavar(R), Bayer) was the first approved inhibitor targeting the vascular endothelial growth factor (VEGF) family kinases, which include VEGFR1, VEGR2, and VEGFR3. Sorafenib was originally approved for the treatment of renal cell carcinoma (RCC) in 2005, hepatocellular carcinoma in 2007, and locally recurrent or metastatic thyroid carcinoma refractory to radioactive iodine treatment in 2013. Six other approved inhibitors with VEGFRs as the main targets are sunitinib (Sutent(R), Pfizer) for RCC, soft tissue sarcoma, thyroid cancer,metastatic pancreatic tumors, gastrointestinal stromal tumor, and several other types of carcinomas; pazopanib (Votrient(R), GlaxoSmithKline) for RCC, soft tissue sarcoma, and thyroid cancer; axitinib (Inlyta(R), Pfizer) for RCC,thyroid cancer, and aplastic anemia, as well as T315I-mutant Bcr–Abl1-driven leukemia; regorafenib (Stivarga(R), Bayer) for gastrointestinal stromal tumors and colorectal cancer; nintedanib (Ofev(R), Boehringer Ingelheim) for the non-oncological indication of idiopathic pulmonary fibrosis; and lenvatinib (Lenvima(R), Eisai Inc.) for RCC and different types of thyroid cancers. Sunitinib, pazopanib, and lenvatinib bind to the “DFG-in”conformation of VEGFRs, while axitinib, regorafenib, and nintedanib bind to inactive VEGFRs adopting the “DFG-out”conformation.
Brand name
Nexavar (Bayer HealthCare); Xarelto (Bayer HealthCare).
Biological Activity
Broad-spectrum kinase inhibitor.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $34.00/50mg |
VIP2Y
|
TargetMol Chemicals Inc.
|
2026-03-06 | |
| $34.00/50mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2026-03-06 | |
| $63.00/500mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-04-30 | |
| $/ |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-04-29 | |
| $1.00/1kg |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-13 | |
| $0.00/10g |
HangZhou RunYan Pharma Technology Co.,LTD.
|
2024-09-11 | ||
| $0.00/1KG |
VIP3Y
|
Hangzhou Hyper Chemicals Limited
|
2024-07-18 | |
| $45.00/1kg |
Hebei Zhuanglai Chemical Trading Co.,Ltd
|
2024-05-31 | ||
| $0.00/1kg |
Nanjing Fred Technology Co., Ltd
|
2023-12-26 | ||
| $10.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-08-24 |
- Since: 2014-12-17
- Address: Zhengzhou High tech Zone, Henan Province, China
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com




 China
China