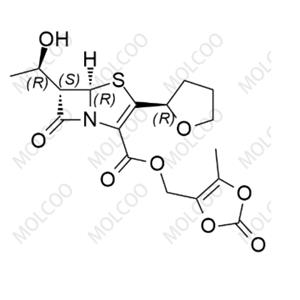
Product Details
| Product Name: Faropenem Daloxate | CAS No.: 141702-36-5 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 10000000 | Release date: 2025/07/31 |
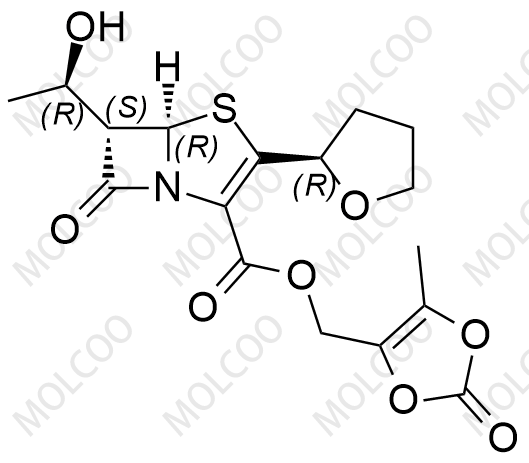
Faropenem Esters Impurity Reference Standards – Accelerating Drug Development & Quality Control
Product Overview
Faropenem Esters, derivatives of Faropenem, are penem-class antibiotics that inhibit bacterial cell wall synthesis, making them effective against drug-resistant infections. To meet the needs of drug R&D, quality analysis, and bioequivalence studies, we offer high-purity Faropenem Esters impurity reference standards, including popular impurities like Impurity 2 (CAS: 106560-13-8), Impurity 18 (CAS: 1690457-78-3), and Impurity 7 (CAS: 120705-68-2).
Key Features
High Purity: HPLC purity ≥95%, with select batches reaching 98%+. COA reports, HNMR, MS, and HPLC spectra provided.
Comprehensive Structural Verification: IR, UV, and 2D NMR (COSY, NOESY, HMBC, HMQC) data for unambiguous impurity identification.
In-Stock Availability: Multiple packaging options (10mg–100g) with expedited shipping for urgent requests.
Regulatory Support: Impurity source analysis, QC strategy development, and analytical method optimization services.
Applications
Drug Discovery: Impurity structural elucidation, metabolite profiling, and degradation pathway studies.
Quality Control: Impurity limit determination, stability testing, and lot-to-lot consistency evaluation.
Bioequivalence: Impurity spectrum comparison for generic drug development.
Technical Expertise
Over a decade of experience in impurity R&D, with custom synthesis capabilities.
Advanced SFC purification technology for complex impurity isolation.
End-to-end solutions including process scale-up and CMC optimization.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $328.00/50mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-11-10 | |
| $328.00/50mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-11-04 | |
| $0.00/1g |
HangZhou RunYan Pharma Technology Co.,LTD.
|
2024-11-21 | ||
| $1.00/1g |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-05-07 | |
| $1.00/1g |
VIP6Y
|
Career Henan Chemical Co
|
2020-01-09 |








 China
China