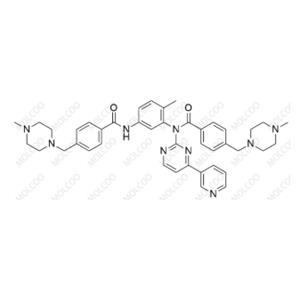
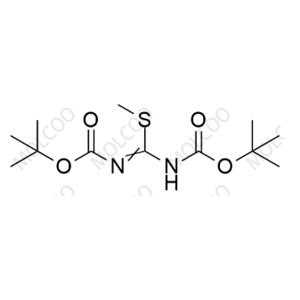
Product Details
| Product Name: Imatinib Impurity 53 | CAS No.: 1356565-46-2 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/07/31 |
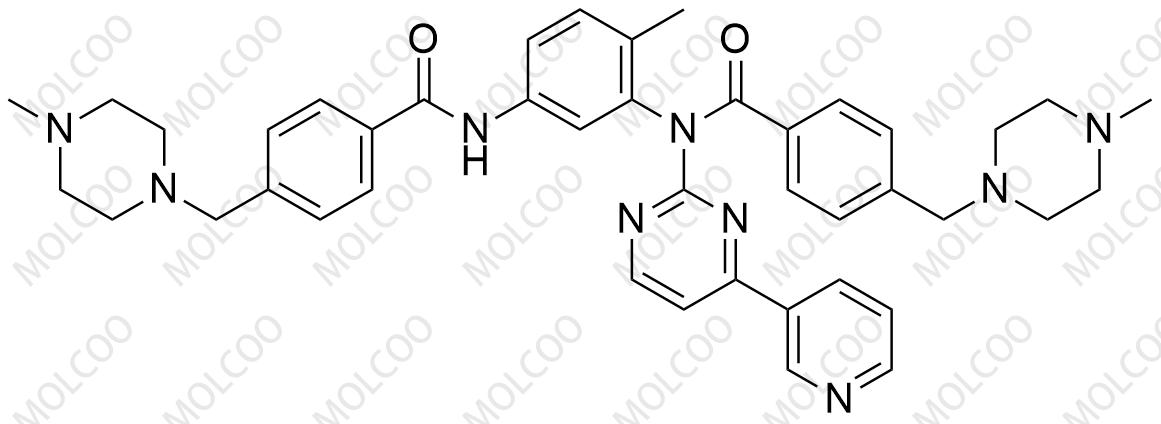
Product Overview
Imatinib impurity reference standards are critical control materials for pharmaceutical R&D, production, and quality assurance. They cover various potential impurities of Imatinib (Imatinib Mesylate), including Impurity A to I. These standards are supplied by GMP-compliant manufacturers with COA certificates and analytical data (HNMR, MS, HPLC), ensuring high purity and structural accuracy for drug registration and testing purposes.
Product Features
Comprehensive Coverage: Includes key impurities from Imatinib synthesis (e.g., piperidine-N-oxide, pyridine-N-oxide) for total quality control.
High Purity: Purity ≥95%, verified by NMR, MS, and other techniques for accurate quantification.
Regulatory Compliance: Meets GMP and pharmacopeia standards for drug submission and research.
Quality Control Methods
Quantitative Analysis: HPLC or LC-MS with correction factors (e.g., Impurity A factor=1.2) to adjust response variability.
Sensitivity: Detection limits as low as 0.004 μg/mL and quantification limits up to 0.014 μg/mL for trace impurity detection.
Method Validation: Precision (RSD ≤2% for consecutive injections) and recovery (80%-120%) tested for reliability.
Packaging & Storage
Packaging: Available in 10 mg, 25 mg, 50 mg, 100 mg vials, sealed and labeled with batch details.
Storage: 2-8℃ (long-term) or 15-30℃ (short-term), protected from light and moisture.
Applications
Pharmaceutical R&D: Impurity profiling of Imatinib APIs and formulations.
Quality Control: Routine impurity testing during drug manufacturing.
Regulatory Support: Complies with global pharmacopeial requirements for reference standards.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-23 | |
| $0.00/25Kg/Bag |
VIP5Y
|
Sinoway Industrial co., ltd.
|
2023-10-18 | |
| $999.00/10kg |
VIP2Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-08 | |
| $1.10/1g |
VIP5Y
|
Dideu Industries Group Limited
|
2021-07-06 |






 China
China