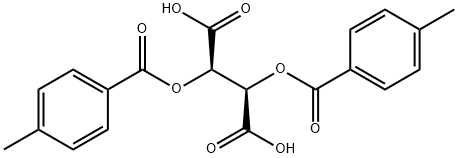
(-)-Di-p-toluoyl-L-tartaric Acid: Chiral Extraction and Hydrogenation Applications
Aug 28,2025
(-)-Di-p-toluoyl-L-tartaric Acid is incompatible with oxidizing agents and can cause skin and eye irritation. This paper conducts a multidimensional study on (-)-Di-p-toluoyl-L-tartaric acid (DTTA), covering its role in the enantiomeric biphasic recognition chiral extraction of mandelic acid, its luminescence and magnetic behaviour when forming complexes with rare earth ions, and its application in palladium-catalysed asymmetric hydrogenation of simple ketimines.

Biphasic recognition chiral extraction of (-)-Di-p-toluoyl-L-tartaric acid
Distribution behavior of mandelic acid enantiomers was studied in the extraction system with (-)-Di-p-toluoyl-L-tartaric acid (D-(+)-DTTA) in organic phase and β-CD derivatives in aqueous phase, and the influence of the types and concentrations of extractants and pH on extraction efficiency was investigated. Hydroxypropyl-β-cyclodextrin (HP-β-CD), hydroxyethyl-β-cyclodextrin (HE-β-CD), and methyl-β-cyclodextrin (Me-β-CD) have stronger recognition abilities for S-mandelic acid than those for R-mandelic acid, among which HP-β-CD has the strongest ability. (-)-Di-p-toluoyl-L-tartaric acid preferentially recognizes R-mandelic acid. pH and the concentrations of extractants have great effects on chiral separation ability. A high enantioseparation efficiency with a maximum enantioselectivity of 1.527 is obtained at pH of 2.7 and the ratio of 2:1 of [(-)-Di-p-toluoyl-L-tartaric acid] to [HP-β-CD]. The obtained results indicate that the biphasic recognition chiral extraction is of stronger chiral separation ability than the monophasic recognition chiral extraction. It may be very helpful to optimize the extraction systems and realize the large-scale production of pure enantiomers.[1]
(-)-Di-p-toluoyl-L-tartaric acid exhibiting luminescence and magnetic behaviors
For the past few years, we have been working on Ln-CPs constructed by aromatic polycarboxylate ligands. As the following studies on this system, in the present work, we focus on novel Ln-CPs employing a chiral and flexible ligand, namely, (-)-Di-p-toluoyl-L-tartaric acid (H2DTTA). This flexible dicarboxylic acid has two equal chiral carbon atoms, and the variable coordination modes provided by this carboxyl-rich molecule can lead to the isolation of metallo-organic hybrid topologies with multiple metal centers. Moreover, to the best of our knowledge, lanthanide complexes of d-H2DTTA enantiomer are practically unexplored, thus offering a good opportunity to exploit the chiral, optical and magnetic properties in view of the generation of new materials. In conclusion, three chiral coordination polymers featuring 1D spiral chain structures were prepared by Ln3+ ions (Ln = Tb, Dy and Ho) and (-)-Di-p-toluoyl-L-tartaric acid. Ditoluoyl-tartrate displays cis-conformation, and acts as a 1,4-dicarboxylate linker in the complexes. The benzoylation of 2- and 3-hydroxyl groups can hinder the coordination of hydroxyl and avoid forming of complicated entangled structures. The single crystal structures and the CD spectra confirm the chirality of the complexes. [2]
Palladium-Catalyzed Asymmetric Hydrogenation of Simple Ketimines
The preparation of chiral amines such as (-)-Di-p-toluoyl-L-tartaric acid from the transition metal-catalyzed asymmetric hydrogenation or transfer hydrogenation of prochiral imines represents one of the most direct and efficient approaches that open a straightforward route to valuable building blocks for natural products, pharmaceuticals and other fine chemical compounds. A variety of chiral metal catalysts has been successfully applied to this reaction over the last few decades. (-)-Di-p-toluoyl-L-tartaric acid gave the highest ee (68%). It is noteworthy that the L-configuration of DTTA matches with the (S)-configuration of the axial chiral bisphosphine ligands, and the configuration of the product is controlled by chiral palladium catalysts. The effect of the amount of activator L-DTTA on the activity and ee was investigated, slightly low enantioselectivity was observed when the amount of L-DTTA was reduced to 10 mol%, gratifyingly, the hydrogenation reaction proceed smoothly with the best 68% ee in the presence of 20 mol% (-)-Di-p-toluoyl-L-tartaric acid.[3]
No catalytic activity was observed for the (R,R)-Me-DuPhos ligand. (R)-C4-TunePhos gave the the highest ee (75%). A decrease of the reaction temperature does not enhance the enantioselectivity. To the ketimines (0.25 mmol) and D-DTTA (20 mol%, 0.05 mmol) was added this catalyst solution, and then the mixture was transferred to an autoclave. The autoclave was stirred at room temperature for 16 h. After release of the hydrogen, the autoclave was opened and saturated NaHCO3 solution was added. The organic phase was separated and the aqueous layer was extracted with CH2Cl2. The combined organic phases were dried over Na2SO4, concentrated under reduced pressure, and purified by flash chromatography on silica gel to yield the corresponding product (-)-Di-p-toluoyl-L-tartaric acid. Therefore, the optimized conditions are: Pd(OCOCF3)2/(R)-C4-TunePhos/D-DTTA/TFE.In summary, using a catalytic amount of a Brønsted acid as activator of simple imines, the highly enantioselective homogeneous Pd-catalyzed asymmetric hydrogenation of simple ketimines was successfully developed with up to 95% of (-)-Di-p-toluoyl-L-tartaric acid. Our ongoing experiments are focused on the asymmetric hydrogenation of other substrates and investigations of the reaction mechanism.
References
[1]Tang, Kewen et al. “Biphasic recognition chiral extraction: A novel method for separation of mandelic acid enantiomers.” Chirality vol. 21,3 (2009): 390-5. doi:10.1002/chir.20601
[2]Han MR, Zhang HT, Wang JN, Feng SS, Lu LP. Three chiral one-dimensional lanthanide-ditoluoyl-tartrate bifunctional polymers exhibiting luminescence and magnetic behaviors. RSC Adv. 2019 Oct 10;9(55):32288-32295. doi: 10.1039/c9ra06920h. PMID: 35530802; PMCID: PMC9072961.
[3]Zhou, X.-Y., Bao, M., & Zhou, Y.-G. (2010). Palladium-Catalyzed Asymmetric Hydrogenation of Simple Ketimines Using a Br?nsted Acid as Activator. Advanced Synthesis & Catalysis, 353(1), 84-88. https://doi.org/10.1002/adsc.201000554
- Related articles
- Related Qustion
- What is (-)-Di-p-toluoyl-L-tartaric acid? Feb 12, 2020
(-)-di-p-toluoyl-l-tartaric acid can be used as a chiral resolving agent for the resolution of the racemic bases to isolate the (-)-enantiomeric forms.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringIvabradine hydrochloride is a selective heart rate–reducing agent used in angina, with novel transdermal delivery formulations under study.....
Aug 28,2025Antineoplastic agents(-)-Di-p-toluoyl-L-tartaric acid
32634-66-5You may like
(-)-Di-p-toluoyl-L-tartaric acid manufacturers
- (-)-Di-p-toluoyl-L-tartaric acid
-

- $0.00 / 25kg
- 2025-11-14
- CAS:32634-66-5
- Min. Order: 1kg
- Purity: 99%min
- Supply Ability: 10000kg
- (-)-Di-p-toluoyl-L-tartaric acid
-

- $0.00 / 25KG
- 2025-07-25
- CAS:32634-66-5
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10000KGS
- (-)-Di-p-toluoyl-L-tartaric acid
-
- $0.00 / 1kg
- 2025-04-04
- CAS:32634-66-5
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton