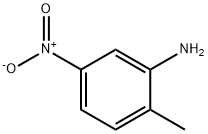
2-Methyl-5-nitroaniline: Synthesis, Biological Activity, and Crystalline Properties
Jun 10,2025
2-Methyl-5-nitroaniline has also been synthesized by the nitration of ortho-toluidine and the monoreduction of 2,4-dinitrotoluene with alcoholic ammonium sulfide. It can be prepared by the electrolytic reduction of ortho-nitrotoluene to ortho-toluidine sulfate and subsequent nitration. Several processes have been reported for the preparation of nitrotoluidines, including 2-Methyl-5-nitroaniline. These include the reaction of nitrocresol with aqueous ammonia, the catalytic hydrogenation of aromatic nitro compounds in the presence of Raney copper, reacting aromatic nitro compounds with hydrogen sulfide in the presence of ammonia dissolved in dioxane, reacting 2,4-dinitrotoluene with hydrogen sulfide in a pyridine solution, and reacting 2,4-dinitrotoluene with carbon monoxide in the presence of cupric oxide and manganese dioxide.

Biological Data of 2-Methyl-5-nitroaniline
Mouse: Groups of 49 or 50 male and 50 female B6C3F, mice, six weeks old, were fed 0.12% or 0.23% (time-weighted average concentration) 2-Methyl-5-nitroaniline (considered to be of high purity) in the diet for 78 weeks and observed for up to 19–20 additional weeks. Groups of 50 males and 50 females served as untreated controls. Mean body weight depression was observed in treated mice of each sex; there was no significant difference in survival between treated and control animals. Rat: Groups of 50 male and 50 female Fischer 344 rats, six weeks of age, were fed 0.005% or 0.01% (time-weighted average concentration) 2-Methyl-5-nitroaniline (considered to be of high purity) in the diet for 78 weeks and observed for up to 30 or 31 additional weeks. Groups of 50 males and 50 females served as untreated controls. A slight depression in mean body weight was observed in high-dose males and in both high- and low-dose females; there was no significant difference in survival between the treated and control groups.[1]
In a screening study in strain A mice, based on the induction of lung tumours, groups of 20 female A/St mice, six to eight weeks of age, received intraperitoneal injections of 25, 50 or 100 mg/kg bw 2-Methyl-5-nitroaniline [purity unspecified] in tricaprylin three times a week for eight weeks. A control group of 80 female mice was untreated and a group of 60 females received intraperitoneal injections of tricaprylin alone. All surviving animals were killed at 16 weeks, when lung adenomas were found in 8% of untreated controls, 11% of tricaprylin-treated controls, 18% of low-dose animals, 30% of mid-dose animals and 6% of high-dose animals. In a similar screening study, groups of 30 male A/J mice, six to eight weeks of age, received intraperitoneal injections of 40, 100 or 200 mg/kg bw 2-Methyl-5-nitroaniline [purity unspecified] in corn oil three times a week for eight weeks. A control group of 20 male mice was untreated and a group of 30 males received intraperitoneal injections of corn oil alone. All surviving animals were killed at 16 weeks. 2-Methyl-5-nitroaniline was mutagenic to several strains of Salmonella typhimurium in the presence and absence of an exogenous metabolic system from Aroclor 1254-induced rat liver, mouse liver or Syrian hamster liver. In one of the studies, positive results were obtained in TA1535 only in the absence of an exogenous metabolic system. 2-Methyl-5-nitroaniline was not mutagenic to Escherichia coli WP2uvrA in the presence or absence of an exogenous metabolic system.
Theoretical Investigation on 2-Methyl-5-nitroaniline Crystals
The nonlinear optical (NLO) properties of some organic materials have received renewed attention in the past years. However, if the crystal structure is centrosymmetric, NLO properties are lost in the solid. Packing then plays a crucial role from the viewpoint of some technological applications. With all this in mind, we decided to study some electrostatic and topological properties of the theoretically determined electron densities of m-nitroaniline (mNA) and 2-methyl-5-nitroaniline (2M5NA) crystals. 2M5NA crystallizes in space group P21/n, with one molecule in the asymmetric unit. The three-dimensional structure has been described in terms of polar chains running along [101̅] and assembled head-to-tail to form nonpolar (101) layers. Connection between adjacent chains within a layer is established through a pair of molecules related by an inversion center. Among the self-consistent set of critical points characterized in the PC electron density of 2-Methyl-5-nitroaniline, 15 are unique intermolecular (3, −1) critical points (ICPs). Each molecule is linked through 27 bond paths with its 13 nearest neighbors. To test to what extent PC results are biased by the multipole model, the B3LYP periodic charge densities of mNA and 2M5NA crystals were projected onto the multipole model. The same set of reciprocal vectors used in the corresponding experimental study was used for mNA, while for 2-Methyl-5-nitroaniline all unique reflections within the range 0 < sin(θ/λ) < 1.10 Å−1 were included. Refinements were performed on F, with unit weights.[2]
A first conclusion one can draw is that the multipole projection has little influence on the values calculated for mNA (less than 3%), but results are considerably biased in the case of 2-Methyl-5-nitroaniline. AIM values corresponding to the experimental charge density are only available for mNA, dipole moment modulus agreeing within 5% with the PC value, but only within 8% with the value obtained after multipole projection of the PC density.The wave function of the reference molecule in this mean electric field was then obtained, and a new superposition density was generated. Resulting AIM values of the molecular dipole moment components and modulus of the reference molecule in mNA, 2M5NA, 2M4NA, and pNA crystals are reported. But what deserves additional consideration is the good performance of the apparently rough uniform electric field approximation. To further test its validity, for mNA and 2-Methyl-5-nitroaniline the procedure described above was carried through again, this time employing a nonuniform electric field. This field was defined as the superposition of the field generated by a set of point charges located at the atomic positions of the neighboring molecules in the cluster and a new uniform field. A set of intermolecular bond paths in reasonable agreement with its experimental counterpart was characterized in the B3LYP/6-31G** electron density of mNA and 2-Methyl-5-nitroaniline crystals, this fact revealing that, in spite of its limitations with regard to the electron correlation treatment, the B3LYP hybrid method leads to a reasonable description of the rather low intermolecular electron density, at least as concerns the nature and relative importance of the intermolecular interactions at play.
References
[1]IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Flame Retardants and Textile Chemicals, and Exposures in the Textile Manufacturing Industry. Lyon (FR): International Agency for Research on Cancer; 1990. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 48.) 5-Nitro-ortho-Toluidine.
[2]Fantoni, Adolfo C et al. “On the electron density topology and electrostatic properties of nitroanilines. A theoretical investigation on m-nitroaniline and 2-methyl-5-nitroaniline crystals.” The journal of physical chemistry. A vol. 113,34 (2009): 9527-32. doi:10.1021/jp904318q
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringTris(2-aminoethyl)amine is a water soluble tripodal ligand used to modify metal oxides for catalysis and as a scaffold for stable collagen mimetics.....
Jun 10,2025Chemical Materials2-Methyl-5-nitroaniline
99-55-8You may like
2-Methyl-5-nitroaniline manufacturers
- 2-Methyl-5-nitroaniline
-

- $10.00 / 1KG
- 2025-12-11
- CAS:99-55-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 2-methyl-5-nitroaniline
-

- $0.00 / 200kg
- 2025-11-19
- CAS:99-55-8
- Min. Order: 20kg
- Purity: 99%
- Supply Ability: 20 tons
- 2-Methyl-5-nitroaniline
-
- $5.00 / 1KG
- 2025-09-25
- CAS:99-55-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available