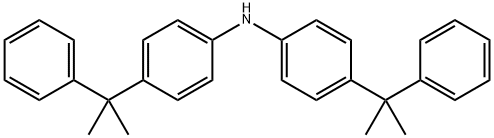
Bis[4-(2-phenyl-2-propyl) phenyl]amine: synthesis and correlational study
Dec 12,2025
Introduction
Bis[4-(2-phenyl-2-propyl) phenyl]amine (Figure 1),also known as antioxidant KY-405, is a crucial aromatic amine antioxidant. It is widely utilized to protect natural rubber and synthetic rubbers—including styrene-butadiene, isoprene, chloroprene, and butyl rubbers—against aging induced by heat, light, and ozone. Additionally, it exhibits excellent synergistic effects when combined with sulfur-containing antioxidants. Simultaneously, this antioxidant is well-suited for light-colored and colored rubber products, and demonstrates particularly outstanding thermal aging resistance when applied in chloroprene rubber colored cable sheaths.
![Figure.1.Bis[4-(2-phenyl-2-propyl)phenyl]amine.jpg Figure.1.Bis[4-(2-phenyl-2-propyl)phenyl]amine.jpg](/NewsImg/2025-11-29/6390003602808618552087063.jpg)
Bis[4-(2-phenyl-2-propyl) phenyl]amine synthesis
Bis[4-(2-phenyl-2-propyl)phenyl]amine was prepared using diphenylamine as raw material and α-methylstyrene as alkylation reagent through F-C alkylation reaction. The effect of reaction conditions on the yield of the product was investigated. It has been shown that the optimum conditions of the reaction is that the reaction temperature is 90°C, reaction time 6h, the molar ratio of α-methylstyrene to diphenylamine being 2.4∶1, using zinc chloride as catalyst. The yield of the product can be 87.0 %. Product was characterized by NMR. The melting point of product is 100.1~100.5°C. Pilot process experiment indicates that the process is suitable for industrial production.[1]
Effects of Bis[4-(2-phenyl-2-propyl) phenyl]amine on the Refined Oriental Lacquer
Oriental lacquer is a natural polymeric material with a water in oil (W/O) emulsion sap, which is obtained by tapping Rhus trees, specifically Rhus succedanea, found in Taiwan and Vietnam. The sap of raw oriental lacquer must be refined by stirring under a temperature below 45℃ to reduce the water content to around 3% or other refined processes for shortening the curing time and improving the gloss and other film properties of the raw oriental lacquer. It is defined as a refined oriental lacquer (ROL). The ROL film possesses a wax-like gloss and has elegant beauty and high durability compared to synthetic coatings and is widely used for wood furniture and handicraft finishing in Taiwan. However, the poor lightfastness of ROL is a major limitation and it must be improved for the widespread utilization of ROL as a natural coating.The poor lightfastness of ROL results from the main component, catechol derivatives,such as urushiol, laccol, or thitsiol, of Rhus vernicifera (grown in China and Japan), R.succedanea (in Taiwan and Vietnam), and Melanorrhoea usitata (in Thailand and Myanmar)saps, respectively. Many photo-stabilizers, such as UV screeners, UV absorbers, hindered amine lightstabilizers (HALS), antioxidants, singlet oxygen scavengers, and excited state quenchers,can be used for reducing the photo-degradation of polymer films. Hong et al. added HALS and a benzotriazole UV absorber to an oriental lacquer to enhance its photostabilization.
In this study, two experiments were performed. First, five types of antioxidants, including three primary antioxidants, such as 2,2'-methylenebis(6-nonyl-p-cresol) (coded as AO-1), 2,2'-methylenebis(6-tert-butyl-4-methylphenol) (AO-2), and bis[4-(2-phenyl-2-propyl) phenyl]amine, and two secondary antioxidants, such as tris (2,4-ditert-butylphenyl) phosphite (AO-P) and dilauryl thiodipropionate (AO-S), were investigated to determine which is the most effective for improving the lightfastness of ROL. Secondly, the appropriate quantity of the best antioxidant, including 0, 1, 2, 3, 5, and 10 phr, was also determined. The lightfastness parameters, such as brightness difference (ΔL*), yellowness difference (ΔYI), and color difference (ΔE*), as well as other coating and film properties, were assessed. The results showed that the primary antioxidants had higher efficiency than secondary antioxidants for improving the lightfastness of ROL. Among the primary antioxidants, the 5 phr bis[4-(2-phenyl-2-propyl) phenyl]amine was the most effective at improving the lightfastness of ROL; however, 1 phr addition had already shown significantly improved efficiency. In addition, the drying time of ROL was extended and film properties decreased when increasing the content of bis[4-(2-phenyl-2-propyl) phenyl]amine, but the 1-phr-containing ROL displayed superior film properties, especially adhesion and bending resistance, compared with the raw ROL film.[2]
Distribution, Partitioning and Bioaccumulation of Antioxidants
Substituted diphenylamine antioxidants (SDPAs) and benzotriazole UV stabilizers (BZT-UVs), previously under reported classes of organic contaminants, were determined in sediment, water, and freshwater biota in an urban creek in Canada. SDPAs and BZT-UVs were frequently detected in all matrices including upstream of the urban area in a rural agricultural/woodlot region, suggesting a ubiquitous presence and bioaccumulation of these emerging contaminants. Spatial comparisons were characterized by higher levels of SDPAs downstream compared with the upstream, implying a possible influence of the urban activities on the antioxidant contamination in the sampling area. In sediment, bis[4-(2-phenyl-2-propyl) phenyl]amine, dioctyl-diphenylamine (C8C8), and dinonyl-diphenylamine (C9C9) were the most dominant congeners of SDPAs, with concentrations up to 191 ng/g (dry weight, d.w.). Benthic invertebrates Crayfish (Orcoescties spp.) had larger body burdens of SDPAs and BZT-UVs compared to pelagic fish (hornyhead chub (Nocomis biguttatus) and common shiner (Luxilus cornutus)) in the creek and partitioning coefficients demonstrated that sediment was the major reservoir of these contaminants. This is the first report of bioaccumulation and partitioning behaviors of SDPAs and BZT-UVs in freshwater environments.[3]
References
[1]Su JH,et al. Preparation Process Research & Development on AntioxidantBis[4-(2-phenyl-2-propyl)phenyl]amine[J].Guangdong Chemical Industry,2016,43(09):76-78.
[2]Lu KT, Lee JJ. Effects of Adding Antioxidants on the Lightfastness Improvement of Refined Oriental Lacquer. Polymers (Basel). 2021;13(7):1110. Published 2021 Mar 31. doi:10.3390/polym13071110
[3]Lu Z, De Silva AO, Peart TE, et al. Distribution, Partitioning and Bioaccumulation of Substituted Diphenylamine Antioxidants and Benzotriazole UV Stabilizers in an Urban Creek in Canada. Environ Sci Technol. 2016;50(17):9089-9097. doi:10.1021/acs.est.6b01796
- Related articles
- Related Qustion
Kuromanin chloride has many physiological functions such as anti-oxidation, anti-tumor, and neuroprotection, which is widely used in food, pharmaceutical etc.....
Dec 12,2025Plant extracts3-Chloro-1-(N,N-dimethyl)propylamine is used primarily as an industrial and research organic chemical intermediate acting as an alkylating reagent.....
Dec 12,2025Pharmaceutical intermediatesBis[4-(2-phenyl-2-propyl)phenyl]amine
10081-67-1You may like
Bis[4-(2-phenyl-2-propyl)phenyl]amine manufacturers
- Bis[4-(2-phenyl-2-propyl)phenyl]amine
-
![10081-67-1 Bis[4-(2-phenyl-2-propyl)phenyl]amine](/ProductImageEN/2022-09/Small/87156654-64a7-40b5-a383-7272d1a3a659.jpg)
- $0.00 / 1KG
- 2025-12-11
- CAS:10081-67-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Bis[4-(2-phenyl-2-propyl)phenyl]amine
-
![10081-67-1 Bis[4-(2-phenyl-2-propyl)phenyl]amine](/ProductImageEN1/2025-03/Small/f024f965-bb0b-439c-8238-23ca3cb6128b.gif)
- $0.00 / 25kg
- 2025-12-01
- CAS:10081-67-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- Bis[4-(2-phenyl-2-propyl)phenyl]amine
-
![10081-67-1 Bis[4-(2-phenyl-2-propyl)phenyl]amine](/ProductImageEN/2022-05/Small/06151634-39dc-407f-a1a1-4c15610347e3.jpg)
- $1.10 / 1g
- 2025-11-18
- CAS:10081-67-1
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons