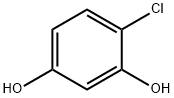
4-Chlororesorcinol: From Cosmetic Use to Environmental Degradation Studies
Aug 25,2025
Abstract
4-Chlororesorcinol is widely applied as a coupler in hair dye formulations and as a reagent in spectrophotometric analyses. Recent studies extend its significance to environmental research, where electrochemical oxidation achieves effective mineralization in wastewater treatment, and photolysis experiments reveal its unique degradation pathways in aqueous systems.
Hair Dye Formulations
4-Chlororesorcinol is a halogenated phenol that is used as a hair colorant in over 30 hair dye and color products, generally at concentrations < 2.5%. It reacts with primary intermediates to form the final dye-stuff. The coupling-reaction can be accelerated by addition of an oxidizing agent (e.g. hydrogen peroxide), but can also be achieved by air oxidation. [1] [2]
Coupling Agent
Simple and accurate spectrophotometric method for the estimation of benzocaine (BENZ) as pure form and in its formulation (ear drops) in aqueous solution has been developed. The method is based on the diazotization of BENZ, with equivalent amount of nitrite, in an acidic medium to yield the diazotized benzocaine. Then the diazotized benzocaine is coupled with 4-chlororesorcinol (4-CRL) reagent in basic medium to formed, an intense yellow azo dye, which is water-soluble and it has good stability. The yellow azo dye exhibits maximum absorption at 436 nm. The relationship between absorbance and concentration gave good range of determination from 10 to 50 μg. BENZ in final volume of 10 ml i.e.,1 to 5 μg.ml-1 with a molar absorptivity and Sandell's sensitivity index values of 3.722104 l.mol-1. cm-1 and 0.0044 μg.cm-2 respectively, a relative error of –1.06 to +2.47% and a relative standard deviation was not more than 0.921% depending on the concentration level of BENZ, low detection limit of 0.1924 μg.ml-1 and low of quantitation value equal to 0.6416 μg.ml-1have been estimated. The method has been applied to the estimation of BENZ in ear drop (otocol drops). [3]

Applications in wastewater treatment
Electrochemical treatment of wastewaters containing 4-chlororesorcinol
The electrochemical oxidation of aqueous wastes polluted with 4-chlororesorcinol has been studied on boron-doped diamond electrodes on acidic medium. The voltammetric results showed that in the potential region where the supporting electrolyte is stable, reactions occur, resulting in the loss of activity due to electrode fouling. Galvanostatic electrolysis study showed that the oxidation of these wastes in single-compartment electrochemical flow cell with boron doped diamond anodes deal to the complete mineralization of the organics but is no indication of electrode fouling. Resorcinol, 1,2,4-trihydroxybenzene, benzoquinone, maleic, fumaric, and oxalic acids have been detected as soluble organics and chlorides (Cl−) and hypochlorites (ClO−) as mineral products during the electrolysis of 4-chlororesorcinol. The electrochemical oxidation of 4-chlororesorcinol consists of a sequence of steps: Release of Cl and/or hydroxylation of the aromatic ring; formation of quinonic compounds; oxidative opening of aromatic ring to form carboxylic acids; and oxidation of carboxylic acids to carbon dioxide. Both, direct oxidation at boron doped diamond surface and mediated oxidation by powerful oxidants electrogenerated from electrolyte oxidation at anode surface are involved in these stages.[4]
Photolysis of 4-chlororesorcinol in water
Research on photoinduced reactions of halogenated phenols is of interest for environmental photochemistry and for synthetic organic chemistry. Previous studies have uncovered interesting mechanistic features, including ring contraction from benzene to cyclopentadiene from orthoderivatives and two-electron processes forming carbocations and carbenes from para derivatives. In the present work, the study of the aqueous photochemistry of 4-chlororesorcinol (1), which combines the conformational properties of both types of derivatives, using nanosecond transient absorption spectroscopy and photoproduct analysis. The absorption spectra obtained upon pulsed laser excitation of 1 showed the occurrence of both o-Cl and p-Cl elimination, the first observed transients being the ketene 3-hydroxy-6-fulvenone (2, λmax = 255 nm) and the carbene 2-hydroxy-4-oxo-2, 5-cyclohexadienylidene (3, λmax = 405 and 395 nm). The reactivities of 2 and 3, the spectra of the secondary transients and the analysis of the final products showed that the two HCl elimination pathways take place concurrently. Most probably, the bifurcation step is the competition between intersystem crossing on the molecular level and o-Cl elimination on the singlet surface; p-Cl elimination proceeds on the triplet surface. Remarkably, the quantum yield of p-Cl elimination from 1 is lower by one order of magnitude compared to that found in para-halogenated phenols, while that of o-Cl elimination from 1 is comparable to ortho-halogenated phenol. To explain this result, a suggestion proposes that o-Cl elimination is the major deactivation step, forming an intermediate singlet cation which is able to recombine to ground state 1, thereby limiting the observed photochemical quantum yields. [5]
Reference
[1] Andersen, F. A. (1996). Final report on the safety assessment of 4-Chlororesorcinol. J. Am. Coll. Toxicol., 15, 284-294.
[2] Scientific Committee on Consumer Safety (SCCS). (2010). Opinion on 4-chlororesorcinol (COLIPA No. A12) (SCCS/1224/09, rev. 12 July 2010). European Commission. Retrieved from https://health.ec.europa.eu/document/download/f6635185-f033-49c8-b6eb-de8330c0b120_en?
[3] Mohammed, M. M., Othman, N. S., & Al-Abady, F. M. (2021). Using of 4-Chlororesorcinol as a Coupling Agent in Spectrophotometric Determination of Benzocaine. Rafidain Journal of Science, 30(3), 1-11.
[4] Nasr, B., & Abdelatif, G. (2009). Electrochemical treatment of wastewaters containing 4‐chlororesorcinol using boron doped diamond anodes. The Canadian Journal of Chemical Engineering, 87(1), 78-84.
[5] Richard, C., Krajnik, P., & Grabner, G. (2010). Photolysis of 4-chlororesorcinol in water: competitive formation of a singlet ketene and a triplet carbene. Physical Chemistry Chemical Physics, 12(42), 14322-14329.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringSemax, a synthetic peptide (ACTH analog), studies show its neuroprotection via modulating molecules, transcriptome, and inhibiting Aβ aggregation.....
Aug 25,2025Supplements4-Chlororesorcinol
95-88-5You may like
4-Chlororesorcinol manufacturers
- 4-Chlororesorcinol
-

- $0.00 / 1KG
- 2025-11-27
- CAS:95-88-5
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- 4-Chlororesorcinol
-

- $1.00 / 1KG
- 2025-11-27
- CAS:95-88-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 4-Chlororesorcinol
-

- $0.00 / 25KG
- 2025-06-27
- CAS:95-88-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg