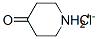4-oxopiperidinium chloride: Synthesis and Application
Dec 6,2022
General description
4-oxopiperidinium chloride, also known as 4-peridone hydrochloride, 4-oxydoperidone hydrochloride can be used for the preparation of 1-tert-butyloxycarbonyl-4-peperidone. 1-tert-butyloxycarbonyl-4-peperidone is a very important intermediate of medicine, pesticides and other chemical additives.
Synthetic routes

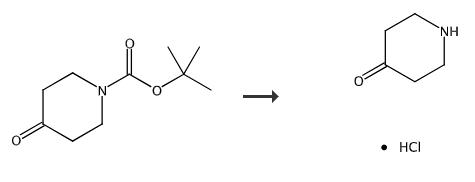
Fig. 1 The synthetic method 1 of 4-oxopiperidinium chloride.
To a solution of tert-butyl 4-oxopiperidine-1-carboxylate (2300 g, 11.6 mol) in 1,4-dioxane was added a solution of HCl (g)/1,4-dioxane (4 L, 10 mol/L) slowly at 0°C. After the addition, the reaction mixture was stirred for 4 h and TLC (EtOAc/petroleum ether = 1:5) showed the reaction was complete. The solvent was removed in vacuo to afford piperidin-4-one hydrochloride as a brown solid [1].

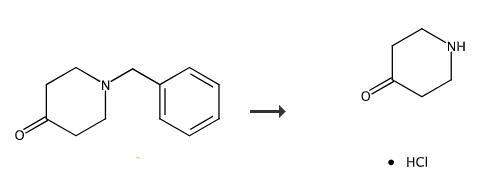
Fig. 2 The synthetic method 2 of 4-oxopiperidinium chloride.
A representative procedure for the cleavage of 1-benzyl-4-α -(cyaiKjbenzylidene)-piperidine 10a is as follows: To a solution of 10a (1.26 g, 4.38 mmol) in methylene chloride (15 ml) was added α -chloroethyl chloroformate 2 (0.62 ml, 5.69 mmol) slowly at 0 °C. The reaction mixture was stirred for 20 min at 0°C After removal of the solvent. After removal of die solvent, the carbamate 10b was dissolved in methanol and refluxed for 40 min. Removal of die solvent ami trituration with ether afforded 4-α -(cyanobenzylidene)-piperidine hydrochloride 10c as a white solid in 90 % yield. Compound 11c, yield 85% [2].
Application
As an intermediate in organic synthesis
In this paper, regiospecific, double intraannular C-N bond cleavages of N-alkyl 4-oxopiperidinium salts (4-oxopiperidinium chloride derivative) are presented. The reaction sequence involves a charge-transfer complex, in situ formed between sulfonyl chloride and N-methylmorpholine, which induces S-Cl bond homolysis of sulfonyl chloride, yielding a reactive sulfonyl radical that further induces the double C-N bond cleavages of N-alkyl 4-oxopiperidinium salt. The secondary amine thus produced was trapped by sulfonyl chloride to yield the desired sulfonamide product. The key feature of this protocol is that two intraannular C-N bonds of the 4-oxopiperidine ring are cleaved in one step under metal- and oxidant-free conditions [3].
In the title compound 3,5-bis(4-methoxybenzylidene)-1-methyl-4-piperidone, C22H23NO3, (I), the central heterocyclic ring adopts a flattened boat conformation, while in the related salt 3,5-bis(4-methoxybenzylidene)-1-methyl-4-oxopiperidinium chloride (4-oxopiperidinium chloride derivative), C22H24NO3+.Cl-, (II), the ring exhibits a 'sofa' conformation in which the N atom deviates from the planar fragment. The pendant benzene rings are twisted from the heterocyclic ring planes in both molecules in the same direction, the range of dihedral angles between the ring planes being 24.5 (2)-32.7 (2)degrees. The dominant packing motif in (I) involves centrosymmetric dimers bound by weak intermolecular C-H...O hydrogen bonds. In (II), cations and anions are linked by strong N-H...Cl hydrogen bonds, while weak C-H...O and C-H...Cl hydrogen bonds link the cations and anions into a three-dimensional framework [4].
A new organic compound 3, 5-diethyl-2, 6-di(4-methoxyphenyl)-4-oxopiperidinium chloride (DMOC, 4-oxopiperidinium chloride derivative) was synthesized and its crystals were grown from an ethanolic solution adopting slow evaporation solution growth technique. The structure of the grown crystal was analyzed by single crystal X-ray diffraction study. The compound crystallizes in the orthorhombic system with space group Pnma. H-1 and C-13 Fourier transform nuclear magnetic resonance spectra of DMOC were recorded to elucidate its molecular structure. UV-vis-NIR spectral study showed that the grown crystal is transparent in the entire visible region. The thermal stability of DMOC was studied by thermogravimetric and differential thermal (TG-DTA) analyses [5].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
References
[1] Young J, Czako B, Altman M, et al. Pyridazinones as tyrosine kinase inhibitors and their preparation and use in the treatment of cancer[P]. PCT Int. Appl., 2011084402, 2011.
[2] Yang B V, O'ROURKE D, Li J. Mild and selective debenzylation of tertiary amines using α-chloroethyl chloroformate[J]. Synlett, 1993 (3): 195-196.
[3] Fu Y, Li M P, Shi C Z, et al. Double C–N bond cleavages of N-alkyl 4-oxopiperidinium salts: access to unsymmetrical tertiary sulfonamides[J]. Organic & Biomolecular Chemistry, 2019, 17(48): 10172-10177.
[4] Nesterov V N. 3, 5-Bis (4-methoxybenzylidene)-1-methyl-4-piperidone and 3, 5-bis (4-methoxybenzylidene)-1-methyl-4-oxopiperidinium chloride: potential biophotonic materials[J]. Acta Crystallographica Section C: Crystal Structure Communications, 2004, 60(11): o806-o809.
[5] Yuvaraj V, Jauhar R O M U, NizamMohideen M, et al. Synthesis, structural, optical and thermal studies on 3, 5-diethyl-2, 6-di (4-methoxyphenyl)-4-oxopiperidinium chloride[J]. Journal of Molecular Structure, 2016, 1123: 238-244.
- Related articles
- Related Qustion
Some research suggests that SAMe is more effective than placebo in treating mild-to-moderate depression and is simply as effective as antidepressant medications without the side effects.....
Dec 5,2022Biochemical Engineering9, 9-Bis (methoxymethyl) fluorene can be used as an intermediate in the fields of materials or drug molecules through polymerization reactions or functional groups.....
Dec 7,2022Organic Synthesis Intermediate4-oxopiperidinium chloride
41979-39-9You may like
4-oxopiperidinium chloride manufacturers
- 4-oxopiperidinium chloride
-

- $999.00/ kg
- 2025-12-10
- CAS:41979-39-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- 4-oxopiperidinium chloride
-

- $568.00 / 25kg
- 2025-03-28
- CAS:41979-39-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5666
- 4-Oxopiperidinium chloride
-

- $88.00 / 1KG
- 2024-01-18
- CAS:41979-39-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100000T