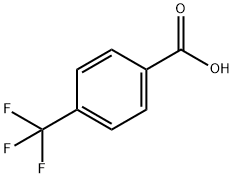
4-(Trifluoromethyl)benzoic Acid: Synthesis, Antifungal Esters, and Analytical Applications
Jun 27,2025
4-(Trifluoromethyl)benzoic acid is a biochemical reagent used in life science research. It has a melting point of 219-220 °C and is often utilized in the synthesis of various organic compounds. This compound is characterized by its trifluoromethyl group, which enhances its biological activity and solubility in organic solvents. 4-(Trifluoromethyl)benzoic acid was used in the synthesis of salicylanilide 4-(trifluoromethyl)benzoates via N,N′-dicyclohexylcarbodiimide coupling in dry N,N-dimethylformamide. It was used as internal standard during the ultra trace analysis of fluorinated aromatic carboxylic acids by GC/MS method.

Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic acid
The spread of fungal infections has recently been increasing because of the eminent presence of the predisposing risk factors, e.g., malnutrition, invasive surgical procedures, treatment with broad-spectrum antibiotics, corticosteroids or other immunosuppressive agents, immunodeficiency related to diseases like diabetes mellitus or human immunodeficiency virus infections. Mycoses may either be superficial or systemic. Based on these facts, we synthesised a new series of salicylanilide esters with 4-(Trifluoromethyl)benzoic acid (illustratively, esters of imidazole derivatives with 3- and 4-(trifluoro-methyl)benzoic acids expressed some activity against Candida strains) and evaluated them as well as their parent salicylanilides as potential antifungal agents. Salicylanilides and their esters with 4-(Trifluoromethyl)benzoic acid were obtained by two procedures used previously by our group. Firstly, salicylanilides were prepared by the reaction of appropriate salicylic acids and anilines in the presence of PCl3 in chlorobenzene (Ph-Cl) under microwave irradiation. Then they were esterified by 4-(Trifluoromethyl)benzoic acid via N,N′-dicyclohexylcarbodiimide (DCC) coupling in dry N,N-dimethylformamide (DMF). Yields of salicylanilides ranged within 75–90%, of esters within 49–86%.[1]
In general, the most antifungal active salicylanilide assayed was N-(4-bromophenyl)-4-chloro-2-hydroxybenzamide. Bromine and 4-(Trifluoromethyl)benzoic acid moiety-containing salicylanilides expressed mostly a significant activity towards T. asahii, A. corymbifera and T. mentagrophytes, while dichlorosalicylanilides were only active against the last strain. It is difficult to postulate distinct structure-activity relationships due to a lot of partially inactive derivatives; for some strains the activity is only sporadic. Similarly, it was shown that the individual biological impacts of related compounds with various substitution patterns may be in part often influenced by the volume of the substituents. This bulkiness can be expressed, e.g., by a bulk parameter like MR (molar refractivity). The esterification of salicylanilides by 4-(Trifluoromethyl)benzoic acid led to the larger molecules with a calculated increment of MR of 34.74. However, as pointed out previously, this modification did not improve the activity unequivocally. Similarly, anilines containing bromine (MR = 7.6) showed a higher antifungal activity than those substituted by chlorine (4.8), fluorine (−0.4) and it is slightly superior to a trifluoromethyl group (MR = 4.0). These data indicate, analogously to lipophilicity, that there is no simple linear correlation between biological activity and steric parameter MR.
When compared to other recently evaluated salicylanilide esters, 4-(Trifluoromethyl)benzoic acid ester did not surpass the activity of salicylanilide acetates, although some MICs are comparable. Contrarily, benzoates are much less potent in vitro than the here presented esters. The comparison with salicylanilide pyrazinoates and esters with N-benzyloxycarbonyl amino acids is not unambiguous, while these derivatives showed more consistent activity; however, MICs of many 4-(trifluoromethyl)benzoates exceeded them. Esters with N-acetyl-L-phenylalanine showed less and less uniform antifungal potency. In summary, we have synthesized nineteen salicylanilides and eighteen corresponding esters with 4-(trifluoromethyl)benzoic acid. New compounds were characterised and all derivatives were evaluated as potential antimycotic agents towards eight fungal strains. It was not possible to determine MICs of a range of the esters because of a low solubility and/or a precipitation in the testing medium. Salicylanilides and their esters affected the fungal growth from 0.49 µmol/L; however, their activity is not uniform and especially against Candida spp. it seems to be most likely sporadic.
References
[1]Krátký M, Vinšová J. Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid. Molecules. 2012 Aug 7;17(8):9426-42. doi: 10.3390/molecules17089426. PMID: 22871645; PMCID: PMC6268247.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCeftiofur has a stable β-lactam ring, which is not easily destroyed by drug-resistant bacteria and can act on β-lactamase-producing Gram-positive and Gram-negative bacteria.....
Jun 27,2025API4-(Trifluoromethyl)benzoic acid
455-24-3You may like
- Diosgenin:Uses,Functions and Synthesis
Dec 12, 2025
- Biosynthesis of Cyclopamine from Cholesterol
Dec 10, 2025
- Synthesis of ribociclib
Dec 10, 2025
4-(Trifluoromethyl)benzoic acid manufacturers
- 4-(Trifluoromethyl)benzoic acid
-

- $0.00 / 1KG
- 2025-12-13
- CAS:455-24-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- 4-(Trifluoromethyl)benzoic acid
-

- $5.00 / 25kg
- 2025-12-12
- CAS:455-24-3
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 100mt/year
- 4-(Trifluoromethyl) benzoic acid
-

- $45.00 / 1KG
- 2025-12-12
- CAS:455-24-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 300MT/year