A JAK2 inhibitor: Fedratinib
Jan 19,2024
Description
Fedratinib is a JAK2 inhibitor approved for treating adult patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The current standard of care is ruxolitinib; patients who have not had success with this treatment or have become intolerant to ruxolitinib currently have a generally poor prognosis[1].
Fedratinib is an ATP-mimetic molecule that binds to both the ATP binding site and the substrate binding site of the target kinase. The drug is not specific to the Janus kinase family. It likely affects many other kinases, resulting in toxicity concerns. Fedratinib was approved as an orphan drug with priority review designation. Still, it contains a black box warning for encephalopathy, proposed to be due to inhibiting neuronal thiamine uptake. Fedratinib was initially discovered at TargeGen, which was acquired by Sanofi-Aventis in 2010. In 2016, fedratinib was acquired by Impact Biomedicines, now a subsidiary of Bristol Myers Squibb.
Chemistry
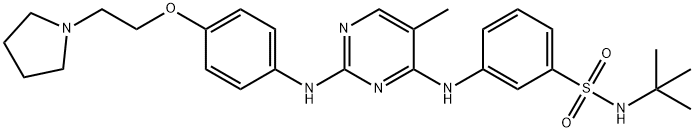
Fedratinib (SAR-302503; TG-101348) is a kinase inhibitor with a 615.62 g/mol molecular weight. The empirical formula is C27H36N6O3S·2HCl·H2O, while the chemical name is N-tert-butyl-3-[(5-methyl-2-{[4-(2pyrrolidin-1-ylethoxy)phenyl]amino}pyrimidin-4-yl)amino]benzenesulfonamide dihydrochloride monohydrate. It is available in gelatin capsules for oral administration.
Synthesis method
![N-(1,1-Dimethylethyl)-3-[[5-methyl-2-[[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]amino]-4-pyrimidinyl]amino]benzenesulfonamide N-(1,1-Dimethylethyl)-3-[[5-methyl-2-[[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]amino]-4-pyrimidinyl]amino]benzenesulfonamide](/NewsImg/2024-01-19/6384127895608784026547878.jpg)
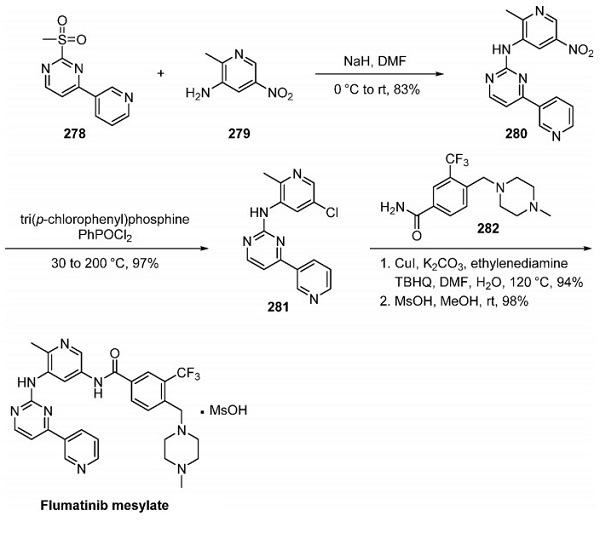
Although no explicitly described large-scale drug synthesis has been reported, a TargeGen patent disclosed a discovery route to fedratinib. The two-step assembly of fedratinib involved a Pd-catalyzed amination of aryl bromide 269 with aminopyrimidine 270[2]. Alternatively, a substitution reaction between aniline 271 and chloropyrimidine 272 allows for the production of identical aryl chloride 273. Subjection of aryl chloride 273 to aniline 274 in the presence of base followed by treatment with acidic isopropanol furnished fedratinib as the bis(HCl) salt hydrate.
Previous methods have been synthesizing aniline 274: alkylation of phenol 275 with alkyl chloride 276 followed by hydrogenation of 277 furnishes aniline 274 in good yield.
![N-(1,1-Dimethylethyl)-3-[[5-methyl-2-[[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]amino]-4-pyrimidinyl]amino]benzenesulfonamide N-(1,1-Dimethylethyl)-3-[[5-methyl-2-[[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]amino]-4-pyrimidinyl]amino]benzenesulfonamide](/NewsImg/2024-01-19/6384127901296642957090178.jpg)
Pharmacokinetics and metabolism
In 10–680 mg doses, fedratinib is rapidly absorbed without food, though low- and high-fat meals increased AUC and Cmax by 24% and 14%, respectively. The time to maximum concentration is 2–4 hours following administration. Fedratinib is ≥90% plasma protein bound with steady-state concentrations achieved after 15 days and an apparent volume of 1770 L[3]. Fedratinib is metabolized by CYP3A4, CYP2C19, and flavin-containing monooxygenase-3, though 80% of circulating drug is unmodified fedratinib. Elimination of radiolabeled fedratinib is 77% through fecal excretion, with only 5% expelled in urine. The effective half-life of fedratinib is 41 h, with an apparent clearance of 13 L/h in MF patients. There was no observed impact of sex, age, body weight, or mild-moderate hepatic impairment on the pharmacokinetics of fedratinib. Fedratinib levels increased 1.5- and 1.9-fold in the setting of moderate (creatinine clearance [CrCl], 30–59 cc/min) and severe (CrCl 15–29 cc/min) renal dysfunction, respectively, and dose reduction for to 200 mg is recommended in patients with CrCl 15–29 cc/min.
References
[1] Blair, Hannah A. . "Fedratinib: First Approval." Drugs 79.11(2019):1719-1725.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
[3] Jan Philipp Bewersdorf. “Beyond Ruxolitinib: Fedratinib and Other Emergent Treatment Options for Myelofibrosis.” Cancer Management and Research (2019): 10777–10790.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringFlumatinib mesylate, also known as HHGV-678, is a selective inhibitor of the tyrosine kinase BCR-ABL1. The drug was discovered and developed by Jiangsu Hengrui Pharmaceutical Group.....
Jan 19,2024InhibitorsFedratinib
936091-26-8You may like
- Fedratinib
-
- $45.00 / 5mg
- 2025-12-17
- CAS:936091-26-8
- Min. Order:
- Purity: 99.96%
- Supply Ability: 10g
- Fedratinib
-

- $0.00 / 1g
- 2025-09-11
- CAS:936091-26-8
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 50kg/Month
- Fedratinib(SAR302503, TG101348)
-

- $200.00 / 1g
- 2024-11-01
- CAS:936091-26-8
- Min. Order: 10g
- Purity: 98%+
- Supply Ability: 100kgs