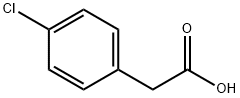
An Overview of 4-Chlorophenylacetic Acid
Aug 21,2025
Abstract
4-Chlorophenylacetic acid is an aromatic carboxylic acid with applications in pharmaceutical synthesis and environmental studies. Understanding its synthesis, metabolic intermediates, and microbial degradation provides a foundation for its use in drug development and environmental remediation.
Introduction
4-chlorophenylacetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is substituted by a 4-chlorophenyl group. It has a role as a xenobiotic metabolite. It is a monocarboxylic acid and a member of monochlorobenzenes. As a substituted phenylacetic acid, 4-chlorophenylacetic acid can be used as a flavoring agent, bactericide, and plant growth regulator. As an intermediate in organic synthesis, it can be utilized to produce penicillin drugs, broad-spectrum organophosphorus insecticides, herbicides, and other compounds.[1][3]

Synthesis
There are many methods for synthesizing 4-chlorophenylacetic acid. A classical approach involves reacting substituted benzyl chlorides with NaCN to form substituted benzyl nitriles which are then hydrolyzed under acidic or basic conditions to yield the 4-chlorophenylacetic acid. Another widely studied method is the carbonylation route, in which substituted benzyl chlorides react with CO to produce arylacetic acids. In most cases, this method employs low-temperature, low-pressure carbonylation catalysts, such as complexes with transition metals like Rh, Re, Ir, Pt, Pd, Co, or Ni as active centers. Phase-transfer catalysts used include quaternary ammonium salts, quaternary phosphonium salts, crown ethers, and polyethylene glycols. 4-chlorophenylacetic acid can also be synthesized by the hydrolysis reaction of p chlorobenzyl cyanide in the alkaline aqueous solution with the presence of C6H 5CH2(CH3)3N+Cl- as phase-transfer catalysts.[2]
Intermediate of Phenylacetates
With the many bioactive axial ligands that have been studied with platinum, a naturally occurring auxin substance called phenylacetic acid (PAA) caught our interest. Apart from being a fundamental block of multiple pharmaceutical intermediates, PAA is also a promising non-toxic drug for cancer treatment. It was suggested that by preserving the aromatic ring and carboxylic group of PAA while adding other structural functionality, more potent derivatives might be generated.
Amongst the selected axial ligands, 4-CPA was of primary interest because previous studies highlighted its effectiveness against estrogen-induced mammary tumorigenesis. Estrogen and its receptor are critical factors in both normal breast development and the progress of breast carcinomas. Further evidence suggests that estrogen production in breast tissues is mainly due to the overexpression of aromatase enzymes, especially in the case of postmenopausal breast cancer. Aromatase is crucial in estrogen biosynthesis, as it catalyses the aromatization of androgen to form estrogen. Increased aromatase enzyme activity is also found in tumour-expressing breast cancer cells, suggesting such enzymes are viable therapeutic targets. Treating estrogen-sensitive breast cancer with current therapies is challenging, as resistance develops after prolonged administration and can involve complications. From this, Sidell et al. studied the efficacy of 4-CPA as a potent aromatase inhibitor and demonstrated a novel mechanism of action by which it antagonizes estrogen signalling. Interestingly, this differs from the mechanism of action of classical anti-breast cancer drugs such as tamoxifen. A prototype platinum(IV) prodrug bearing 4-CPA is expected to express the attributes of both moieties and provide evidence for the effectiveness of this strategy for treating estrogen-sensitive breast cancer.[1]
Degradation of 4-chlorophenylacetic acid
Pseudomonas sp. strain CBS3 was able to utilize 4-chlorophenylacetic acid as the sole source of carbon and energy. When this strain was grown with 4-chlorophenylacetic acid, homoprotocatechuic acid was found to be an intermediate which was further metabolized by the meta-cleavage pathway. Furthermore, three isomers of chlorohydroxyphenylacetic acid, two of them identified as 3-chloro-4-hydroxyphenylacetic acid and 4-chloro-3-hydroxyphenylacetic acid, were isolated from the culture medium. 4-Hydroxyphenylacetic acid was catabolized in a different manner by the glutathione-dependent homogentisate pathway. Degradation enzymes of both of these pathways were inducible.[3]
Reference
[1] Aputen, A. D., Elias, M. G., Gilbert, J., Sakoff, J. A., Gordon, C. P., Scott, K. F., & Aldrich-Wright, J. R. (2022). Bioactive Platinum(IV) Complexes Incorporating Halogenated Phenylacetates. Molecules, 27(20), 7120.
[2] ZENG Zhao kun;PANG Zheng zhi. Synthesis and characterization of p-chlorophenylacetic acid[J]. Journal of Beijing University of Chemical Technology, 2001, 28(1): 41-44.
[3] Klages U, Markus A, Lingens F. Degradation of 4-chlorophenylacetic acid by a Pseudomonas species. J Bacteriol. 1981 Apr;146(1):64-8.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDiethylene glycol methyl ethyl ether has broad industrial relevance and significant potential for future development.....
Aug 21,2025API4-Chlorophenylacetic acid
1878-66-6You may like
4-Chlorophenylacetic acid manufacturers
- 4-Chlorophenylacetic acid
-
- $33.00 / 1kg
- 2025-11-27
- CAS:1878-66-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100kg
- 4-Chlorophenylacetic acid
-

- $10.00 / 1KG
- 2025-11-27
- CAS:1878-66-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 4-Chlorophenylacetic acid
-

- $0.00 / 1KG
- 2025-06-27
- CAS:1878-66-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg