Application research of 2,4,6-triformylphloroglucinol in organic synthesis
Sep 24,2025
Introduction
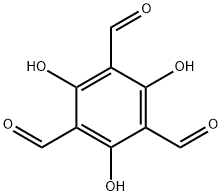
2,4,6-Triformylphloroglucinol (Figure 1) is also known as 1,3,5-Triformylphloroglucinol or 2,4,6-Trihydroxy-1,3,5-benzenetricarboxaldehyde etc. 2,4,6-Triformylphloroglucinol is often used to synthesize covalent organic framework materials because of its symmetrical structure, three active aldehyde groups and three hydroxyl groups. 2,4,6-Triformylphloroglucinol can not only be used to construct framework materials, but also its unique molecular structure has attracted the interest of supramolecular chemists to synthesize a variety of supramolecular structures.This paper mainly enumerates the application research examples in organic synthesis.

A triketoenamine based dynamic covalent network synthesis
Development of thermosets that can be repeatedly recycled via both chemical route (closed-loop) and thermo-mechanical process is attractive and remains an imperative task. In this work, researchers reported a triketoenamine based dynamic covalent network derived from 2,4,6-triformylphloroglucinol and secondary amines. The resulting triketoenamine based network does not have intramolecular hydrogen bonds, thus reducing its π-electron delocalization, lowering the stability of the tautomer structure, and enabling its dynamic feature. By virtue of the highly reversible bond exchange, this novel dynamic covalent bond enables the easy construction of highly crosslinked and chemically reprocessable networks from commercially available monomers. The as-made polymer monoliths exhibit high mechanical properties (tensile strength of 79.4 MPa and Young's modulus of 571.4 MPa) and can undergo a monomer-network-monomer (yields up to 90 %) recycling mediated by an aqueous solution, with the new-generation polymer capable of restoring the material strength to its original state. In addition, owing to its dynamic nature, a catalyst-free and low-temperature reprogrammable covalent adaptable network (vitrimer) was achieved. The design concept reported herein can be applied to the development of other novel vitrimers with high repressibility and recyclability, and sheds light on future design of sustainable polymers with minimal environmental impact.[1]
NH2-UiO-66/COF hybrid material synthesis
The poor stability and low catalytic activity of NH2-UiO-66 in basic solutions require the reactions to be conducted in acidic solutions, which seriously hinders its potential photocatalytic application. Herein, we report that NH2-UiO-66 coated with two-dimensional covalent organic frameworks (COFs) via imine bond connection presents not only high photocatalytic activity but also high stability and adaptability to the solution environment. The NH2-UiO-66/COF hybrid material was fabricated through the Schiff base reaction of NH2-UiO-66 with 4,4',4″-(1,3,5-triazine-2,4,6-triyl)trianiline (TAPT) and 2,4,6-triformylphloroglucinol (TP). The hybrid material showed high stability in an alkaline environment, with only 4.7% of NH2-UiO-66 decomposed after the photocatalytic reaction. The optimum photocatalytic H2 evolution rate was 8.44 mmol/h/g when triethanolamine was used as an electron-donating agent. The results presented here illustrate the possibility for effectively improving both the photocatalytic performance and stability of NH2-UiO-66 by coupling with COFs.[2]
Two beta-keto-enamine-based materials synthesis
Saxitoxin (STX), the most widely distributed neurotoxin in marine waters and emerging cyanotoxin of concern in freshwaters, causes paralytic shellfish poisoning in humans upon consumption of contaminated shellfish. To allow for the efficient monitoring of this biotoxin, it is of high importance to find high-affinity materials for its adsorption. Herein, we report the design and synthesis of a covalent organic polymer for the efficient adsorption of STX. Two β-keto-enamine-based materials were prepared by self-assembly of 2,4,6-triformylphloroglucinol (Tp) with 2,5-diaminobenzoic acid (Pa-COOH) to give TpPa-COOH and with 2,5-diaminotoluene (Pa-CH3) to give TpPa-CH3. The carboxylic acid functionalized TpPa-COOH outperformed the methyl-bearing counterpart TpPa-CH3 by an order of magnitude despite the higher long-range order and surface area of the latter. The adsorption of STX by TpPa-COOH was fast with equilibrium reached within 1 h, and the Langmuir adsorption model gave a calculated maximum adsorption capacity, Qm, of 5.69 mg/g, making this material the best reported adsorbent for this toxin. More importantly, the prepared TpPa-COOH also showed good reusability and high recovery rates for STX in natural freshwater, thereby highlighting the material as a good candidate for the extraction and pre-concentration of STX from aquatic environments.[3]
Organic framework nanosheet synthesis
The development of covalent organic framework nanosheet (COFNS) is becoming a vitally important research field by reason of its high permeability, ordered structure, high utilization of functional site, favourable dispersability and large aspect ratio, resulting in their widespread applications in separation, catalysis, sensing and optical device. In this work, a Tp-Bpy COFNS was prepared via an interfacial synthesis of 2,4,6-triformylphloroglucinol (Tp) and 5,5'-diamino-2,2'-bipyridine (Bpy), which has film morphology, high surface area, large pore, excellent stability and various functional site. It was utilized as a functional material to immobilize aptamers for constructing a sensitive electrochemical aptasensor. Compared with bulk Tp-Bpy COF, Tp-Bpy COFNS can significantly enhance the biosensing performance toward ultra-trace tobramycin. This work is benefit for the exploration of COFNSs and their electrochemical aptasensors in biosensing applications.[4]
References
1.Hu Z, Hu F, Deng L, et al. Reprocessible Triketoenamine-Based Vitrimers with Closed-Loop Recyclability. Angew Chem Int Ed Engl. 2023;62(34):e202306039. doi:10.1002/anie.202306039
2.Wang Y, Yang Q, Yi F, et al. NH2-UiO-66 Coated with Two-Dimensional Covalent Organic Frameworks: High Stability and Photocatalytic Activity. ACS Appl Mater Interfaces. 2021;13(25):29916-29925. doi:10.1021/acsami.1c06008
3.Wang T, Fernandes SPS, Araújo J, Li X, Salonen LM, Espi?a B. A carboxyl-functionalized covalent organic polymer for the efficient adsorption of saxitoxin. J Hazard Mater. 2023;452:131247. doi:10.1016/j.jhazmat.2023.131247
4.Li HK, An YX, Zhang EH, et al. A covalent organic framework nanosheet-based electrochemical aptasensor with sensitive detection performance. Anal Chim Acta. 2022;1223:340204. doi:10.1016/j.aca.2022.340204
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringSodium formaldehyde sulfoxylate,a powerful carcinogen, has been illegally used to whiten wheat foods. Its detection method was reported here.....
Sep 25,2025Food Additives2,4,6-Triformylphloroglucinol
34374-88-4You may like
2,4,6-Triformylphloroglucinol manufacturers
- 2,4,6-Triformylphloroglucinol
-
- $30.00 / 1kg
- 2025-09-25
- CAS:34374-88-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g,kg-tons, free sample is available
- 2,4,6-Triformylphloroglucinol
-
- $30.00 / 1kg
- 2025-09-25
- CAS:34374-88-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g,kg-tons, free sample is available
- 2,4,6-Triformylphloroglucinol
-
- $30.00 / 1kg
- 2025-09-25
- CAS:34374-88-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g,kg-tons, free sample is available