Application research of 4-methoxybenzylamine
Sep 10,2025
Introduction
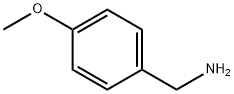
4-Methoxybenzylamine (Figure 1) is an organic compound with the molecular formula C8H11NO. It usually exists in the form of colorless liquid and has a special aromatic smell. 4-Methoxybenzylamine plays an important role in organic synthesis and is often used in the preparation of pharmaceutical intermediates and fine chemicals.

The gramine route to pyrido[4,3-b]indol-3-ones
A novel approach to 3-oxo-γ-carbolines was worked out starting from methyl indol-2-ylacetate via a gramine derivative. After quaternization, ammonia and 4-methoxybenzylamine could be inserted giving appropriate 3-oxo-γ-carbolines. Condensation with 2-chlorobenzaldehyde under microwave irradiation gave a 4-(2-chlorobenzyl)-3-oxo-γ-carboline. N-methylation lead to a product with very promising antifungal and cytotoxic activities.[1]
One-pot synthesis of polyphenolic amino acids
A simple and efficient procedure for the synthesis of N-acyl 4-hydroxy, 4-hydroxy-3-methoxy and 3,4-dihydroxy phenylglycine amides by a strategy based on the multicomponent Ugi reaction is proposed. Hydroxybenzaldehyde derivatives were reacted with 4-methoxybenzylamine, cyclohexyl isocyanide and benzoic acid or 2-naphthylacetic acid to give Ugi adducts that were treated with trifluoroacetic acid yielding N-acyl hydroxyphenylglycine amides in good yields. The same procedure using as acid component protocatechuic acid or hydrocaffeic acid gave N-catechoyl 3,4-dihydroxyphenylglycine amides. The use of N-benzyloxycarbonylglycine as acid component allowed the preparation of a 3,4-dihydroxyphenylglycyl dipeptide derivative. Radical-scavenging activity studies of the polyphenolic amino acid derivatives showed a sharp increase in activity with the increase in number of hydroxyl or catechol groups present. Cyclic voltammetry experiments established a correlation between oxidation peak potentials and the radical-scavenging activity.[2]
Synthesis of Symmetric and Unsymmetric Secondary Amines
The catalytic system generated in situ from the tetranuclear Ru-H complex with a catechol ligand (1/L1) was found to be effective for the direct deaminative coupling of two primary amines to form secondary amines. The catalyst 1/L1 was highly chemoselective for promoting the coupling of two different primary amines to afford unsymmetric secondary amines. The analogous coupling of aniline with primary amines formed aryl-substituted secondary amines. The treatment of aniline- d7 with 4-methoxybenzylamine led to the coupling product with significant deuterium incorporation on CH2 (18%D). The most pronounced carbon isotope effect was observed on the α-carbon of the product isolated from the coupling reaction of 4-methoxybenzylamine (C(1)=1.015(2)). A Hammett plot was constructed from measuring the rates of the coupling reaction of 4-methoxyaniline with a series of para-substituted benzylamines 4-X-C6H4CH2NH2 (X=OMe, Me, H, F, CF3) (ρ=-0.79±0.1). A plausible mechanistic scheme has been proposed for the coupling reaction on the basis of these results. The catalytic coupling method provides an operationally simple and chemoselective synthesis of secondary amine products without using any reactive reagents or forming wasteful byproducts.[3]
Nucleophile crossover experiments
The enantioselective, palladium-catalyzed reaction of benzylamine with (E)-1,3-diphenylallyl ethyl carbonate was examined with 12 different chiral ligands across a range of scaffold types. In 8 out of 12 cases, the observed enantiomeric excess was 36-92% higher when DBU or Cs2CO3 was added. Nucleophile crossover experiments between the N-benzyl-1,3-diphenylallylamine product and 4-methoxybenzylamine mechanistically linked the changes in enantioselectivity to reformation of the η3-allylpalladium intermediate. In the crossover reactions with 9 out of 12 chiral ligands, 10-75% less elimination to 1-phenylbutadiene was observed with Cs2CO3 than with DBU. Analysis of percent crossover vs percent completion of the simultaneous reaction of 1-phenyl-3-methylallyl ethyl carbonate in the crossover experiment revealed that (1) the formation of the 1,3-diphenylallylpalladium intermediate frequently occurred before the reaction of 1-phenyl-3-methylallyl ethyl carbonate was complete, (2) the addition of DBU or Cs2CO3 suppressed formation of the 1,3-diphenylallylpalladium intermediate, and (3) the less polar toluene and THF solvents resulted in less or slower formation of the 1,3-diphenylallylpalladium intermediate than the more polar DCM and DMF solvents.[4]
Effect on aryl-alcohol oxidase
Spectral and catalytic properties of the flavoenzyme AAO (aryl-alcohol oxidase) from Pleurotus eryngii were investigated using recombinant enzyme. Unlike most flavoprotein oxidases, AAO does not thermodynamically stabilize a flavin semiquinone radical and forms no sulphite adduct. AAO catalyses the oxidative dehydrogenation of a wide range of unsaturated primary alcohols with hydrogen peroxide production. This differentiates the enzyme from VAO (vanillyl-alcohol oxidase), which is specific for phenolic compounds. Moreover, AAO is optimally active in the pH range of 5-6, whereas VAO has an optimum at pH 10. Kinetic studies showed that AAO is most active with p-anisyl alcohol and 2,4-hexadien-1-ol. AAO converts m- and p-chlorinated benzyl alcohols at a similar rate as it does benzyl alcohol, but introduction of a p-methoxy substituent in benzyl alcohol increases the reaction rate approx. 5-fold. AAO also exhibits low activity on aromatic aldehydes. 19F NMR analysis showed that fluorinated benzaldehydes are converted into the corresponding benzoic acids. Inhibition studies revealed that the AAO active site can bind a wide range of aromatic ligands, chavicol (4-allylphenol) and p-anisic (4-methoxybenzoic) acid being the best competitive inhibitors. Uncompetitive inhibition was observed with 4-methoxybenzylamine. The properties described above render AAO a unique oxidase. The possible mechanism of AAO binding and oxidation of substrates is discussed in the light of the results of the inhibition and kinetic studies.[5]
References
1. Wollein U, Bracher F. The gramine route to pyrido[4,3-b]indol-3-ones - identification of a new cytotoxic lead. Sci Pharm. 2011;79(1):59-68. doi:10.3797/scipharm.1011-11
2. Monteiro LS, Paiva-Martins F, Oliveira S, Machado I, Costa M. An efficient one-pot synthesis of polyphenolic amino acids and evaluation of their radical-scavenging activity. Bioorg Chem. 2019;89:102983. doi:10.1016/j.bioorg.2019.102983
3. Arachchige PTK, Lee H, Yi CS. Synthesis of Symmetric and Unsymmetric Secondary Amines from the Ligand-Promoted Ruthenium-Catalyzed Deaminative Coupling Reaction of Primary Amines. J Org Chem. 2018;83(9):4932-4947. doi:10.1021/acs.joc.8b00649
4. Leibler IN, Goodstein MB, Easton CA, et al. Reversibility and Enantioselectivity of Palladium-Catalyzed Allylic Aminations: Ligand, Base-Additive, and Solvent Effects. J Org Chem. 2025;90(21):7031-7042. doi:10.1021/acs.joc.5c00606
5. Ferreira P, Medina M, Guillén F, Martínez MJ, Van Berkel WJ, Martínez AT. Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem J. 2005;389(Pt 3):731-738. doi:10.1042/BJ20041903
- Related articles
- Related Qustion
- Synthesis and Application of 4-methoxybenzylamine Jul 19, 2022
4-Methoxybenzenemethanamine is used in the synthesis of COX2 inhibitors based on a pyrimidine scaffold.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGrowth hormone releasing peptide have cytoprotective properties, and their analogs show promise in cancer, metabolism, and regenerative medicine.....
Sep 10,2025API4-Methoxybenzylamine
2393-23-9You may like
4-Methoxybenzylamine manufacturers
- 4-Methoxybenzylamine
-

- $0.00 / 1kg
- 2025-12-13
- CAS:2393-23-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- 4-Methoxybenzylamine
-

- $0.00 / 200KG
- 2025-12-12
- CAS:2393-23-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100mt
- 4-Methoxybenzylamine
-

- $10.00 / 1KG
- 2025-12-11
- CAS:2393-23-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt