Applications and Hazards of UV-328
Aug 27,2025
Characterization
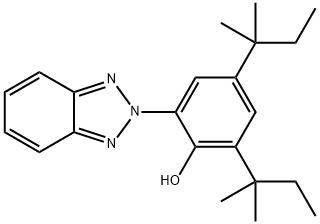
UV-328 is an ultraviolet light absorber (UVA) of the hydroxyphenylbenzotriazole class, which imparts outstanding light stability to plastics and other organic substrates. Additionally, UV-328 is a highly effective light stabilizer for a variety of plastics and other organic substrates. Its use is recommended for the stabilization of styrene homo- and copolymers, acrylic polymers,unsaturated polyesters, polyvinylchloride, polyolefins, polyurethanes, polyacetals, polyvinylbutyral, elastomers, and adhesives. UV-328 features strong UV absorption, low initial color, excellent compatibility in a wide varietyof substrates, good solubility in plasticizers and monomers, and moderately low volatility. It protects polymers as well as organic pigments from UV radiation, helping to preserve theoriginal appearance and physical integrity of molded articles, films, sheets, and fibers duringoutdoor weathering. [1]

The use levels of UV-328 range between 0.10 and 1.0%, depending on substrate and performance requirements of the final application. The product can be used alone or in combination with other additives such as light stabilizers (hindered amines), antioxidants (hindered phenols, phosphites, thiosynergists, hydroxylamines, lactones), and other functionalstabilizers and additives. The use of UV-328 in combination with hindered amine light stabilizers is particularly noteworthy in that a synergistic performance is often observed. Performance data for UV-328 alone and in combination with other additives are available in a variety of substrates. [1]
Hazards and Risks to human health and the environment
UV-328 is characterized by its persistence and its capacity to bioaccumulate and to be long-range transported. Sources of UV-328 in the environment can include industrial facilitiesthat produce or use the substance, wastewater treatment plants, stormwater, landfills and plastic litter/debris. UV-328has been detected in various environment media, including ambient air, water, soil, sediment, biota and humans in many regions of the world. In mammals, the primary health effect of UV-328 is liver toxicity. UV-328 has also been associated with adverse effects on the kidneys in rats, and potential effects on the reproductive system have been suggested instudies on rats and dogs. UV-328 may also lead to anti-androgenic activity based on in vitro study. Finally, UV-328 has been found to be associated with adverse effects in fish. [2]
Degradation Mechanism
UV-328 is one of the dominant BUVSs in fish, human urine, and breast milk. Its can enhanced antiandrogenic activity after metabolism and may potentially cause further adverse effects on sex differentiation in animal systems. Several sexual phenotypes have been reported to be caused by the exposure to androgenic disrupting chemicals. Therefore, future risk assessment of BUVSs asemerging contaminants should take full account of biotransformation products to assess their undesirable biological effect. [3]
By the two hybrid recombinant human androgen receptor yeast bioassay and the in vitro metabolism assay the antiandrogenic activities of UV-328 prior to and after human CYP3A4-mediated metabolic activation/deactivation was investigated. More potent antiandrogenic activity was observed for the metabolized UV-328 in comparison with UV-328 at 0.25 mM ((40.73 ± 4.90) % vs. (17.12 ± 3.00) %), showing a significant metabolic activation. In contrast, the metabolized UV-P at 0.25 mM resulted in a decreased antiandrogenic activity rate from (16.08 ± 0.95) % to (6.91 ± 2.64) %, indicating a metabolic deactivation. Three mono-hydroxylated (OH) and three di-OH metabolites of UV-328 were identified by ultra-performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC-Q-TOF-MS/MS), which were not reported previously. The study further surmised that the hydroxylation of UV-328 occurs mainly at the alicyclic hydrocarbon atoms based on the in silico prediction of the lowest activation energies of hydrogen abstraction from C-H bond. The results for the first time relate antiandrogenic activity to human CYP3A4 enzyme-mediated hydroxylated metabolites of UV-328. The biotransformation through hydroxylation should be fully considered during the health risk assessment of structurally similar analogs of UV-328 and other emerging contaminants. [3]
References:
[1] Data, P. (2014). Technical data sheet. Dimensions (H x W x D), 2000(1,315), x510x815.
[2] https://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-PUB-factsheet-UV328-2023.English.pdf
[3] Zhuang, S., Lv, X., Pan, L., Lu, L., Ge, Z., Wang, J., ... & Zhang, C. (2017). Benzotriazole UV 328 and UV-P showed distinct antiandrogenic activity upon human CYP3A4-mediated biotransformation. Environmental Pollution, 220, 616-624.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringOsimertinib mesylate treats EGFR-mutant NSCLC, shows good efficacy/PFS, targets CNS mets, but faces C797S/MET resistance.....
Aug 27,2025APIYou may like
2-(2H-Benzotriazol-2-yl)-4,6-ditertpentylphenol manufacturers
- UV Absorber UV-328
-

- $1.00 / 1PCS
- 2025-12-11
- CAS:25973-55-1
- Min. Order: 1PCS
- Purity: 99%
- Supply Ability: 10 mt
- 2-(2H-Benzotriazol-2-yl)-4,6-ditertpentylphenol
-

- $0.00 / 25KG
- 2025-12-11
- CAS:25973-55-1
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 200mt/year
- 2-(2H-Benzotriazol-2-yl)-4,6-ditertpentylphenol
-

- $0.00 / 25KG
- 2025-12-11
- CAS:25973-55-1
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month