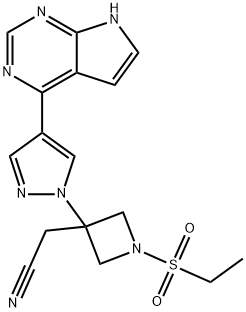
Baricitinib: Clinical Efficacy and Safety
Nov 19,2024
General Description
Baricitinib has shown promising clinical efficacy in treating Alopecia Areata (AA), with significant improvements in hair regrowth observed in patients receiving the medication, particularly at the 4 mg dose, as demonstrated in the BRAVE-AA1 trial. Patients treated with baricitinib exhibited higher proportions of achieving specific improvements in the Severity of Alopecia Tool (SALT) scores, with a greater percentage reaching SALT scores of ≤ 20 by week 36 compared to the placebo group. The medication was effective in reducing hair loss, as indicated by the percent change from baseline in SALT score and higher response rates in SALT50, SALT75, SALT90, and SALT100 categories. In terms of safety, baricitinib demonstrated a consistent and favorable safety profile across various trials, with manageable adverse events and a low frequency of serious adverse events, supporting its use in treating atopic dermatitis.

Figure 1. Baricitinib
Clinical Efficacy1
Baricitinib selectively inhibits JAK1 and 2. Baricitinib exhibited 100-fold selectivity for JAK1 and JAK2 over JAK3. The lower affinity for JAK3 could potentially decrease the immunosuppressive effects expected as a consequence JAK3 inhibition. Baricitinib has an oral bioavailability of approximately 80%. Metabolism is mainly carried out via CYP3A4.The half-life is approximately 12 h. Approximately 75% of the drug is eliminated via urine and 20% via feces. Of the drug eliminated, 69% is unchanged in urine and 15% in feces.
Baricitinib is currently approved for the treatment of moderate to severe rheumatoid arthritis, moderate to severe atopic dermatitis, and more recently coronavirus disease 2019 (COVID-19) in hospitalized adults requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation. In May and June 2022, baricitinib was approved for severe alopecia areata by the EMA and FDA, respectively.
BRAVE-AA1 (NCT03570749), a phase 2/3 adaptive, placebo (PBO)-controlled, double-blind trial, evaluated the efficacy and safety of oral baricitinib in patients with AA who had ≥ 50% scalp hair loss. A total of 110 patients were randomized 1:1:1:1 to receive baricitinib 1 mg (28 patients), 2 mg (27 patients), or 4 mg (27 patients) once daily (OD) or placebo (28 patients). The trial had two interim analysis after all patients completed 12/16 weeks and 36 weeks of treatment or early discontinuation. The first interim analysis (12/16 weeks) aimed to identify two doses of baricitinib to advance to a second phase 3 trial (BRAVE-AA2) and study’s phase 3 part, and the second analysis to assess the drug's efficacy and safety.
Safety2
In BRAVE-AA1, AEs occurred in 59.6%, 50.8%, and 51.3% of patients treated with baricitinib 4 mg, 2 mg, and placebo, respectively. In BRAVE-AA2, AEs occurred in 68.4%, 66.1%, and 63.0% of patients treated with baricitinib 4 mg, 2 mg, and PBO, respectively. The proportions of patients who discontinued the study regimen due to AEs were low and were similar between study groups. Serious AEs occurred in 2.1%, 2.2%, and 1.6% of patients on baricitinib 4 mg, 2 mg, and PBO, respectively, in BRAVE-AA1, and 3.4%, 2.6%, and 1.9%, respectively, in BRAVE-AA2. In both studies, acne and the incidence of urinary tract infections were more common in the baricitinib-treated groups. At least one infection was seen in 31.4% of patients on baricitinib 4 mg, 25.1% on baricitinib 2 mg, and 28.0% on PBO in BRAVE-AA1, and 29.6%, 37.4%, and 29.2% under baricitinib 4 mg, 2 mg, and PBO, respectively, in BRAVE-AA2.
Herpes zoster infections occurred in 0.7%, 0.5%, and 0.5% of patients on baricitinib 4 mg, 2 mg, and PBO, respectively, in BRAVE-AA1, and 1.3%, 1.9%, and 0.6%, respectively. respectively, in BRAVE-AA2. All herpes zoster infections were localized. There were no opportunistic infections, venous thromboembolic events, or gastrointestinal perforations in any of the studies. In BRAVE-AA1, a patient with cardiovascular risk factors suffered a myocardial infarction on baricitinib 2 mg. In BRAVE-AA2, one case of prostate cancer was reported in a patient on a PBO and one case of B-cell lymphoma in a patient on baricitinib 4 mg. In BRAVE-AA1, one patient with a history of gastrointestinal bleeding experienced grade 4 anemia (defined as a hemoglobin level < 6.5 g/dl) when treated with baricitinib 4 mg and had to discontinue the study. In both studies, elevated levels of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol were observed in approximately 25% and 40% of baricitinib-treated patients, respectively. The recently published 52-week data showed that the most frequent treatment-emergent AEs included headache, upper respiratory tract infection, nasopharyngitis, urinary tract infection, COVID-19 infection, acne, and creatine phosphokinase elevation. The frequency of discontinuations due to AEs was low and similar between groups. During the BRAVE-AA1 extension phase, there was one case of herpes zoster, COVID-19, and appendicitis on baricitinib 4 mg, all patients recovered, and none discontinued. During the BRAVE-AA2 extension period, a COVID-19 infection in one patient on baricitinib 4 mg led to study discontinuation. In BRAVE-AA1, squamous cell carcinoma and ductal carcinoma in situ were reported after 16 months and 10 months in a patient on baricitinib 2 mg and 4 mg, respectively. No opportunistic infections, tuberculosis, venous thromboembolism (VTE), gastrointestinal perforations, or deaths were reported in any of the studies during the extension period. Most laboratory changes were similar between the baricitinib groups in both studies and similar to those reported in the 36-week data.
References:
[1] EGíDIO FREITAS T T Emma Guttman Yassky. Baricitinib for the Treatment of Alopecia Areata.[J]. Drugs, 2023, 83 9. DOI:10.1007/s40265-023-01873-w.
[2] AMELIA MELO T T Jose Manuel Carrascosa. Baricitinib for the treatment of atopic dermatitis.[J]. Journal of Dermatological Treatment, 2022. DOI:10.1080/09546634.2021.1967268.
- Related articles
- Related Qustion
- Baricitinib: Pharmacodynamics and pharmacokinetics Jun 7, 2023
Baricitinib is a selective and reversible Janus kinase 1 (JAK1) and 2 (JAK2) inhibitor. Janus kinases belong to the tyrosine protein kinase family.
- The pharmacologic action of Baricitinib Sep 17, 2019
Baricitinib is a selective and reversible Janus kinase 1 (JAK1) and 2 (JAK2) inhibitor. Janus kinases belong to the tyrosine protein kinase family and play an important role in the proinflammatory pathway signalling that is frequently over-
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAmmonium formate shows promise as an electrochemical fuel, but safety concerns arise in animal feed due to formamide presence, requiring further investigation for certain species.....
May 27,2024APIBaricitinib
1187594-09-7You may like
- Baricitinib
-
- $2.00 / 1kg
- 2025-11-25
- CAS:1187594-09-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1200KG/M
- Baricitinib
-

- $5.00/ KG
- 2025-11-25
- CAS:1187594-09-7
- Min. Order: 0.10000000149011612KG
- Purity: 99% hplc
- Supply Ability: 5000kg
- Baricitinib
-

- $5.00/ KG
- 2025-11-25
- CAS:1187594-09-7
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS