Bicine: Good's Zwitterionic Buffer and Copper Corrosion Inhibitor
Sep 18,2025
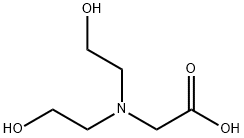
Bicine is a buffering agent used in biochemistry and molecular biology that was selected and described by Good et al. It is a zwitterionic, bis(2-hydroxyethyl)amine buffer that is useful for a pH range of 7.6 – 9.0. It is commonly used in protein crystallization, in the study of enzymatic reactions, and for electrophoresis. It is used in thin layer ion exchange chromatography for protein resolution, as well as a multiphasic buffer system for SDS-PAGE of proteins. It is soluble in water at 0°C up to 1.1 M. Bicine forms a complex with most common metals so stability constants and concentrations should be taken into consideration when choosing this buffer.

Bicine promotes rapid formation of β-sheet-rich amyloid-β fibrils
In the brain of patients afflicted with cerebral amyloid angiopathy (CAA), depositions of amyloid-β (Aβ) are found in the walls of the cortical and leptomeningeal vasculature. While Aβ monomers of various lengths are produced by sequential β- and γ-secretase processing of membrane-bound amyloid-β protein precursors, Aβ40 is the predominant isoform in vascular deposits. Physio-chemical environmental factors, including the pH, temperature, ionic strength, and the presence of cosolvents, influence the nonpolar interactions involved in the intermolecular β-sheet assembly characteristic of amyloid fibrils. Bicine (N,N-Bis(2-hydroxyethyl)glycine) is a zwitterionic buffer from Good’s list, commonly used in biological and biochemical experiments. Here, we report that bicine promotes the formation of β-sheet-rich Aβ40 fibrils in vitro. We investigated the accelerating effect of bicine on the propensity of Aβ40 aggregation in comparison to phosphate buffered saline (PBS), by using thioflavin T (ThT) fluorescence assays, far-ultraviolet circular dichroism (CD) spectroscopy, and transmission electron microscopy (TEM). Time-dependent changes in the size composition of Aβ40 species upon incubation with bicine was monitored via SDS-PAGE followed by photo-induced cross-linking of unmodified proteins (PICUP) and silver staining. Cell viability assays were conducted to assess the toxicity of the aggregated peptides.[1]
Many endeavours to regulate the Aβ misfolding in vitro indicate that biochemical environments highly influence the degree and morphology of protein aggregation. Due to the versatile roles of buffer ions in amyloid fibrillogenesis, adequate buffer selection is crucial for in vitro experiments. In this study, we demonstrate that bicine accelerates the formation of β-sheet-rich fibrils of Aβ40 in vitro. Fibrillation kinetic assays of bicine-treated Aβ40 show that aggregation occurs in a time- and concentration-dependent manner with high reproducibility. Modifying environmental factors can vastly change the propensity of amyloid aggregation. Slow acquisition of fibrillar Aβ40 due to long aggregation periods can be overcome by taking advantage of buffer ions that facilitate fibrillation. Our results show that aggregation conditions with bicine buffer can rapidly acquire β-sheet-rich Aβ40 aggregates that possess toxicity. Although we did not observe any side effects by bicine during the acquirement of Aβ40 fibrils in this study, there is a need to consider the possibility of unwanted side effects before applicating bicine to different protocols.
The adsorption performance of two glycine derivatives as anti-corrosion agents
Copper is an important metal not only for its corrosion resistance, but also for its wide industrial applications, in which the metal itself or its alloys are involved. The corrosion resistance of copper has been attributed to the accumulation of corrosion products on its surface, forming a protecting layer. Intense research efforts are directed to find new environmentally friendly inhibitor compounds for Cu corrosion in seawater media. In this paper, we used bicine and tricine as novel inhibitors to control the corrosion of Cu in aerated 3.5 mass% NaCl solutions. To the best of our knowledge, no report on bicine and/or tricine as a Cu corrosion inhibitor in Cl−-containing media has been published till date. Both materials are water-soluble and have minimal tendency to bind to soil or sediments. They are not expected to be biodegradable but are non-toxic and unlikely to persist in the environment. The corrosion rate and % of inhibition efficiency were determined using OCP, AC and DC electrochemical measurements.[2]
The electrochemical experiments showed that both bicine and tricine could effectively inhibit Cu from corrosion in a 3.5% NaCl solution. Moreover, tricine exhibited better inhibition capability than bicine. To validate the aforementioned data and deeply analyze the influence of the electronic parameters and geometrical structure on the corrosion inhibition performance of bicine and tricine, theoretical calculations and MC simulations were implemented. Bicine and tricine were shown to be effective corrosion inhibitors for Cu corrosion compared to glycine in chloride solutions. The molecules acted like mixed-type inhibitors with inhibition efficiency of ∼98% in the presence of 5 mM tricine. The inhibition process was assigned to the adsorption property through the O atoms and/or the N atoms of the molecules on the active centers of the metal surface by the formation of a barrier film. The adsorption model obeyed the Langmuir adsorption isotherm and the calculated values of ΔGads for both inhibitors on Cu were in the range from −28 to −32 kJ mol−1, which indicated physical adsorption. A quantum simulation was performed by DFT calculations using both the GGA/DNP 4.4 levels.
References
[1]Kim HY, Lee H, Lee JK, Kim HV, Kim KS, Kim Y. Bicine promotes rapid formation of β-sheet-rich amyloid-β fibrils. PLoS One. 2020 Oct 13;15(10):e0240608. doi: 10.1371/journal.pone.0240608. PMID: 33048999; PMCID: PMC7553346.
[2]Elgendy A, Nady H, El-Rabiei MM, Elhenawy AA. Understanding the adsorption performance of two glycine derivatives as novel and environmentally safe anti-corrosion agents for copper in chloride solutions: experimental, DFT, and MC studies. RSC Adv. 2019 Dec 18;9(72):42120-42131. doi: 10.1039/c9ra08617j. PMID: 35542890; PMCID: PMC9076542.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering3-Isocyanatopropyltriethoxysilane has oligomer issues, aids mesoporous organosilica synthesis, and enables selective Hg2? preconcentration.....
Sep 18,2025APIBicine
150-25-4You may like
- Bicine
-

- $0.00 / 25Kg/Drum
- 2025-12-04
- CAS:150-25-4
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 500kgs
- Bicine
-

- $25.00 / 1ASSAYS
- 2025-12-03
- CAS:150-25-4
- Min. Order: 100ASSAYS
- Purity: 99.5%
- Supply Ability: 100 mt
- Bicine
-

- $0.00 / 1removed
- 2025-09-11
- CAS:150-25-4
- Min. Order:
- Purity: 99.72%
- Supply Ability: 10g