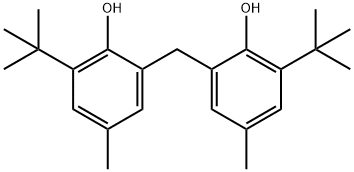
2,2'-Methylenebis(4-methyl-6-tert-butylphenol): Antioxidant Use, Catalytic Degradation, and Toxicological Effects
Sep 22,2025
2,2'-Methylenebis(4-methyl-6-tert-butylphenol) is a high-efficiency nonpolluting antioxidant, applicable to the manufacture of synthetic rubber, latex and natural rubber, being one of excellent phenol Antioxidants. It is applicable to light colored or colored rubber product. In plastic industry, this product can prevent the thermal aging and light aging of polyester chloramine, polystyrene, ABS resin, polyoxymethylene and cellulose resin with the dosage of 0.5-1%. It is a universal strong bisphenol antioxidant, widely used in natural rubber, synthetic rubber, polyethylene, polypropylene, polyoxymethylene, ABS resin, polyether chloride, polyurethane and other synthetic materials. 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) has high thermal stability, good anti-Oxygen effect, no pollution, no coloring, no frost spraying, good oil solubility, non-volatile loss, no effect on rubber vulcanization and plasticity, the role of non-unstable latex and less consumption and other characteristics.

Catalytic ozonation of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol)
The leachate produced from municipal solid waste landfills contains high concentration of refractory organics, ammoniacal-nitrogen and heavy metals. The occurrence, contamination levels, and distribution patterns of SPAs in different Chinese cities were investigated and the observation evidenced that 11 SPAs, including 2,2'-Methylenebis(4-methyl-6-tert-butylphenol), were positively detected in the 56 sewage sludge samples collected from different wastewater treatment plants (Lu et al., 2019). The acute, subchronic and chronic experiments were conducted to elucidate the hazard potential of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol). The results showed that AO 2246 can induce hemorrhagic death in rats. The other significant effect of AO 2246 was atrophy of testicular tubules and decrease of spermatogenesis, accompanied by decrease in testis weigh (
Rodil et al., 2012).Inspired by the above mentioned studies, in present work, we prepared the composite material of nano-Fe3O4 at cow dung ash (nano-Fe3O4@CDA) and investigated the catalytic ozonation of AO 2246 in aqueous solution over the as-prepared nano-Fe3O4@CDA. This study focused on three main aspects: (1) examination of catalytic ozonation performance of nano-Fe3O4@CDA on AO 2246; (2) identification of the intermediates and evaluation of the toxicity of dominant oxidation products of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol); (3) elucidation of the catalytic mechanism and potential pathways in catalytic ozonation.[1]
The mineralization of the pretreated landfill leachate with catalytic ozonation was represented by the TOC removal. 60% of TOC was removed under the same conditions, while the COD removal rate of 78% was obtained. This illustrated that some certain intermediate products of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) remained in the leachate as residual products, which may be toxic for the water matrix. It was most likely that there existed competition between AO 2246 and other contaminants. In this study, we prepared the novel nano-Fe3O4@CDA composite catalyst and demonstrated that 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) can be degraded efficiently by catalytic micro-ozonation using the O3/Fe3O4@CDA system. The GC–MS results showed that 3,5-bis(1,1-dimethylethyl)phenol, 4-(1,5-dihydroxy-2,6,6-trimethylcyclohex-2-enyl)but-3-en-2-one, ethanone, 1-(1,4-dimethyl-3-cyclohexen-1-yl)-, 5-tert-butyl-6-3, 5-diene-2-one, 2-hydroxyhexanoic acid, 2-propenoic acid 1,1-dimethylethyl ester, butanoic acid, 2-methyl-, methyl ester and propanoic acid, 2, 2-dimethyl- were the main products of AO 2246 degradation in the O3/Fe3O4@CDA system. The EPR results and the free radical quenching studies showed that the catalytic ozonation with Fe3O4@CDA resulted in the generation of more •OH, which was responsible for the degradation of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol).
Developmental and neurobehavioral toxicity of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol)
Antioxidants, both natural (such as vitamin A, C, and E) and synthetic, provide protection against the deleterious effects of oxidation and decay. While natural antioxidants are challenging to extract and preserve, synthetic antioxidants, particularly synthetic phenolic antioxidants (SPAs), are widely employed in various fields, including pharmaceuticals, dental materials, and everyday items, due to their established technology, cost-effectiveness, and efficacy. 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) (AO2246) is an important low molecular weight SPA. The total production volume of AO2246 reached 454–4536 tons per year in the United States (Liu and Mabury, 2020). Several studies reported the occurrence of AO2246, which has not received much attention previously, in different environmental matrices, such as 0.00068 μg/L in sewage effluent (China), 0.39 ng/g dry weight (dw) in indoor dust (North America), and 1.7–3.53 ng/g dw in sludge (China). AO2246 has been found to cause high mortality rates and morphological abnormalities in zebrafish (
Yang et al., 2018b). However, the available data is restricted solely to morphological parameters, thereby leaving the effects of 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) on locomotor behavior, neurological function, and the underlying molecular mechanisms inadequately comprehended.[2]
2,2'-Methylenebis(4-methyl-6-tert-butylphenol) has a 96-hour LC50 value of 3 μM. The exposure of AO2246 resulted in a significant reduction in both hatching rate and heart rate. Analysis of locomotor behavior demonstrated that larvae exposed to AO2246 doses exceeding 2 μM exhibited a significant decrease in both total distance and mean velocity. Electrophysiological recordings demonstrated a noteworthy reduction in spike activity at a concentration of 3 μM, relative to control conditions. The administration of AO2246 at 3 μM elicited morphological reactivity and immune alteration of the midbrain microglia in the macrophage/microglia-transgenic zebrafish line, indicating a potential contribution of neurological disorders to behavioral defects. RNA sequencing analysis revealed altered gene expression profiles at high 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) concentrations, particularly the dysregulation of pathways associated with neuronal function. The present study demonstrates that AO2246 exposure elicits developmental and neurobehavioral toxicity in zebrafish larvae. Specifically, exposure to 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) was found to cause disturbances in neuronal electrophysiological activity and neurological disorders, which ultimately led to the impairment of locomotor behavior in zebrafish larvae.
Subchronic and chronic toxicity studies of 2,2'-methylenebis(4-methyl-6-tert-butylphenol) in rats
General toxicity studies on 2,2'-methylenebis(4-methyl-6-tert-butylphenol) (MBMBP) were conducted using male and female Wistar rats. LD50 values were greater than 5 g/kg BW by oral administration for both sexes. Diarrhea was observed until 5 days. In the subchronic test, rats were fed diet containing MBMBP at 0, 0.12, 0.6 or 3.0% for 12 weeks. Severe suppression of body weight gain was observed in both sexes of 0.6 and 3.0% groups. Death accompanied by hemorrhage from nasal cavity was observed in 0.6 and 3.0% males and 3.0% females. Dose-dependent toxicity to the liver in both sexes was observed in blood chemical analysis. Histopathologically, testicular atrophy and decrease of spermatogenesis were dose- and time-dependently observed in all treated males. Atrophy of ovaries was evident in 0.6 and 3.0% females. Thymus atrophy and bone marrow hypoplasia were observed in both sexes of 0.6 and 3.0% groups. In the chronic test, rats were fed diet containing MBMBP at 0, 0.01, 0.03 and 0.1% for 18 months. Body weight gain was only suppressed in both sexes receiving 0.1%. Histopathologically, testicular atrophy and decrease of spermatogenesis were apparent in 0.1% males. No neoplastic response by 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) administration was noted. NOAEL was concluded to be 0.03% in the diet (12.7 mg/kg BW/day for male rats and 15.1 mg/kg BW/day for female rats).[3]
References
[1]Ma C, Jia S, Yuan P, He Z. Catalytic ozonation of 2, 2'-methylenebis (4-methyl-6-tert-butylphenol) over nano-Fe3O4@cow dung ash composites: Optimization, toxicity, and degradation mechanisms. Environ Pollut. 2020 Oct;265(Pt B):114597. doi: 10.1016/j.envpol.2020.114597. Epub 2020 Apr 22. PMID: 32806439.
[2]Chai Y, Sheng D, Ji X, Meng Y, Shen F, He R, Ma R, Wang Y. Developmental and neurobehavioral toxicity of 2,2'-methylenebis(6-tert-butyl-4-methylphenol) (antioxidant AO2246) during the early life stage of zebrafish. Sci Total Environ. 2023 Nov 15;899:166306. doi: 10.1016/j.scitotenv.2023.166306. Epub 2023 Aug 14. PMID: 37586501.
[3]Takagi A, Takada K, Sai K, Ochiai T, Matsumoto K, Sekita K, Momma J, Aida Y, Saitoh M, Naitoh K, et al. Acute, subchronic and chronic toxicity studies of a synthetic antioxidant, 2,2'-methylenebis(4-methyl-6-tert-butylphenol) in rats. J Toxicol Sci. 1994 May;19(2):77-88. doi: 10.2131/jts.19.2_77. PMID: 8072042.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringD-cycloserine shows therapeutic potential for psychiatric disorders, autism, and cognitive decline treatments.....
Sep 22,2025DrugsYou may like
2,2'-Methylenebis(4-methyl-6-tert-butylphenol) manufacturers
- 2,2'-Methylenebis(6-tert-butyl-4-methylphenol)
-
- 2025-12-12
- CAS:119-47-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 2,2'-Methylenebis(4-methyl-6-tert-butylphenol)
-

- $0.00 / 1kg
- 2025-12-12
- CAS:119-47-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000kg
- 2,2'-Methylenebis(4-methyl-6-tert-butylphenol)
-

- $0.00 / 1G/KG
- 2025-12-12
- CAS:119-47-1
- Min. Order: 1G/KG
- Purity: 99%
- Supply Ability: 100MT