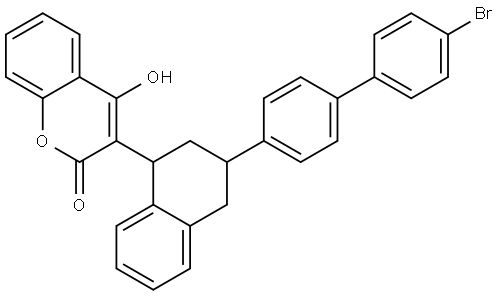
Brodifacoum:Mechanism of Toxicity
Nov 29,2021
Brodifacoum is a 4-hydroxycoumarin anticoagulant that acts as a vitamin K antagonist. It was registered as a pesticide in 1979 in the United States although in 2008 it was made a restricted use pesticide by the Environmental Protection Agency. This means it can only be used by certified pesticide applicators; however, the makers of D-Con which contains 0.005% brodifacoum by weight have challenged this and brodifacoum is currently available in D-Con and in various other pesticide products for the eradication of mice and rats although it is also used on larger mammals such as possums. Brodifacoum currently remains available to the general public.

Use
Brodifacoum is used as a rodenticide (commonly 0.005% by weight).
Mechanism of Toxicity
Brodifacoum has a very low solubility in water and typically
enters water through erosion where it is then found in the
sediment. Brodifacoum concentrations are typically not
measurable in water systems.
Products that contain brodifacoum as pesticide can remain
toxic for a long period of time in the environment. The rate of
decay of brodifacoum depends upon the amount of rainfall. As
the product that contains brodifacoum degrades over time, the
brodifacoum is absorbed into the soil. Soil bacteria degrade
brodifacoum over weeks to months although soil type,
temperature, and the presence of microorganisms that will
degrade brodifacoum all influence the time it takes to degrade.
Environmental Fate
Brodifacoum, like other hydroxycoumarins, interferes with the production of vitamin K–dependent coagulation factors. Vitamin K is a cofactor for the carboxylation of specific glutamic acid groups in coagulation factors II (prothrombin), VII, IX, and X. During this step, vitamin K is oxidized to vitamin K 2,3-epoxide. The regeneration of vitamin K by vitamin K 2,3-epoxide reductase is prevented by brodifacoum. As a result, dysfunctional decarboxy-coagulation factors are produced and coagulation is impaired. Brodifacoum is over 100 times more potent than warfarin on a molar basis in rats.
- Related articles
- Related Qustion
Botulism, a disease of the nervous system in animals and humans, was first recorded in Germany in 1735 and was thought to be due to eating a tainted sausage. The name botulism comes from the German ‘botulus’ for sausage. Botulinum toxin (mo....
Nov 29,2021Chemical ReagentsBromobenzene is a colorless, flammable liquid with a density greater than water and with an aromatic odor. It is synthesized by the reaction of bromide with benzene in the presence of iron powder. It is used for organic synthesis, particula....
Nov 29,2021Organic Chemistry