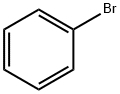
Toxicity of Bromobenzene
Nov 29,2021
Bromobenzene is a colorless, flammable liquid with a density greater than water and with an aromatic odor. It is synthesized by the reaction of bromide with benzene in the presence of iron powder. It is used for organic synthesis, particularly in the production of the intermediate phenylmagnesium bromide. Bromobenzene is an additive to motor oils and used as a crystallizing solvent. Bromobenzene is used as an ingredient in the manufacture of phencyclidine, a recreational drug.
Environmental Fate
Bromobenzene will volatilize from dry surfaces, due to its vapor pressure of 4.18mmHg at 25°C, and therefore will exist as a vapor in the environment. Bromobenzene will undergo little hydrolysis in water and little biodegradation by aquatic microorganisms. Bromobenzene is not expected to adsorb to sediment from water due to its soil sorption constant (Koc) of 150 and water solubility of 446 mg l-1. It is also expected to have a high mobility in soil and volatilize easily from moist surfaces due to its Henry’s law constant of 2.47×10-3atmm3 mol-1 at 25°C. Bioconcentration factors range from low values of 8.8 in carp to moderately high values of 190 in algae.
Mechanism of Toxicity
Liver toxicity is due to reactive metabolites of bromobenzene, such as the epoxide and quinone derivatives, and not due to bromobenzene itself. Reactive metabolites can covalently bind with hepatocellular macromolecules or organelles and may cause decreased hepatocyte oxygen uptake and ATP levels, altered Ca2+ homeostasis, and reduced cytosolic and mitochondrial GSH levels. Increased toxicity is associated more so with the 3,4-epoxide metabolic intermediate rather than the 2,3-epoxide. Hepatic centrilobular necrosis has been observed after the depletion of GSH reserves. For instance, rats that have been administered bromobenzene and diethyl maleate (which depletes GSH) developed extensive liver necrosis, while rats that were administered bromobenzene and cysteine (a GSH precursor) exhibited no necrosis. Also, conjugation of quinone derivatives with GSH can lead to reactive metabolites that can cause toxicity in the kidney.
- Related articles
- Related Qustion
- The Use of Bromobenzene as a Solvent Jan 5, 2024
Bromobenzene is the simplest member of the class of bromobenzenes, it has a role as a non-polar solvent. Bromobenzene shows different bromination selectivity in polar solvents containing ionic liquid.
- Bromobenzene: metabolism and applications Jul 21, 2023
Bromobenzene is a yellow crystalline solid with high toxicity. It has been studied for its metabolism, toxic effects, and use as a flame retardant in polymers.
Brodifacoum is a 4-hydroxycoumarin anticoagulant that acts as a vitamin K antagonist.....
Nov 29,2021Chemical pesticides ?P-methoxybenzaldehyde is a member of the class of benzaldehydes consisting of benzaldehyde?itself carrying a methoxy substituent at position 4. It has a role as an insect repellent, a human urinary metabolite.....
Nov 29,2021Organic ChemistryBromobenzene
108-86-1You may like
- Bromobenzene
-

- $1.00 / 25KG
- 2025-12-03
- CAS:108-86-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Bromobenzene
-
- $100.00 / 1KG
- 2025-09-25
- CAS:108-86-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- bromobenzene
-

- $0.00 / 1kg
- 2025-06-20
- CAS:108-86-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons