Cobalt Carbonyl Complexes: Imidazol-2-ylidene Stabilization and Polymer-Supported Catalysis
Oct 15,2025
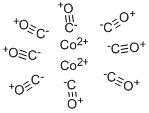
Cobalt carbonyl (known as dicobalt octocarbonyl) is a versatile reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst in many organicconversion reactions, which include hydrogenation, isomerization, hydroformylation, polymerization, and carbonylation.

Imidazol-2-ylidene stabilized tetrahedral cobalt carbonyl complexes
Cobalt complexes are among the most important compounds used in homogeneous processes catalyzed by transition metals. Since the discovery of cobalt catalyzed hydroformylation by Otto Roelen in 1938, millions of tons of carbonyls and alcohols are produced from alkenes, hydrogen gas (H2) and carbon monoxide (CO) each year. Recent reports have also shown NHC stabilized cobalt complexes to be effective precursors for both chemical vapour deposition (CVD) and atomic layer deposition (ALD) in integrated circuit manufacture. Based on the importance and growing interest in the use of NHC stabilized cobalt complexes, this study surveys the number and properties of tetrahedral cobalt carbonyl complexes in the Cambridge structural Database (CSD) with particular emphasis on the Co–CO and Co-Im bond lengths. Global chemical reactivity descriptors such as chemical hardness (η), electronic chemical potential (μ), electrophilicity (ω), ionization energy (I) and the significance of the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Imidazol-2-ylidene stabilized cobalt carbonyl complexes are also studied computationally using the density functional theory (DFT).[1]
A CSD substructure search using ConQuest version 1.20 revealed seventeen tetra-coordinated crystal structures of imidazole-2-ylidene stabilized cobalt carbonyl complexes. The geometry of the coordinated ligands (Im-Co-CO bond angle) around the central cobalt is generally tetrahedral with bond angles ranging from 97.62o to a maximum of 114.83o with an average of 105.3o. The slight differences can be attributed to steric interactions as well as intermolecular interactions within the crystal lattice. Imidazole-2-ylidene stabilized tetrahedral cobalt carbonyl complexes are a group of promising transition metal complexes with varied applications. The σ-donor and π-donor properties of the imidazole ring enhances the stability of the cobalt-carbonyl bond and decreases the high partial pressures of carbon monoxide required for cobalt carbonyl catalyzed processes such as hydroformylation. This paper assessed the stability of tetrahedral imidazole-2-ylidene stabilized this complexes. A substructure search of the Cambridge Structural Database (CSD) version 5.39 plus one update revealed seven (17) crystal structures of cobalt carbonyl complexes with substituted imidazol-2-ylidenes. Generally, there was a positive correlation between the imidazole-2-ylidene-cobalt bond distance and the cobalt-carbonyl bond distance. Implying that a shorter Im-Co bond distance results in a more stable Co–CO bond thereby stabilizing the CO ligand.
Polymer-supported cobalt carbonyl complexes as novel solid-phase catalysts
The synthesis of cyclopentenone derivatives via the cobalt carbonyl-mediated annulation of an alkyne, an alkene and carbon monoxide, the Pauson–Khand (P–K) reaction, was first described in 1971. A synthetically very important process, the P–K reaction is routinely applied using a stoichiometric amount of the transition metal complex. Although the catalytic process was reported as early as 1973, it was confined to the strained reactive alkenes norbornene and norbornadiene. Given our current interest in polymer-supported cobalt complexes and increasing awareness of the environmental and handling advantages conferred by such solid-phase methodologies, we decided to determine the viability of the catalytic P–K reaction using immobilised cobalt carbonyls. Our preliminary investigations, which reveal for the first time that polymer-supported cobalt complexes do indeed catalyse the P–K reaction, are reported herein. The cobalt carbonyl resins to be tested as catalyst precursors were prepared as follows. Reaction of ‘polymer-bound triphenylphosphine with Co2(CO)8 in THF at room temperature generated a resin-bound mixture of phosphine-substituted cobalt carbonyl complexes (ca. 1∶1). The purple resin, which is significantly more air-stable than Co2(CO)8, was characterised on the basis of its IR and P NMR spectra. Heating this resin at 75 °C in 1,4-dioxane cleanly converted it into a second form, assigned the structure of the neutral bisphosphine on the basis of its IR and 31P NMR spectra. Resin, however, can be more conveniently prepared directly from 1 and Co2(CO)8 without prior isolation.[2]
In conclusion, we have shown for the first time that solid-phase cobalt carbonyl complexes have significant potential as catalysts of the P–K reaction. As with all supported catalysts, a very simple work-up procedure is required: filtration of the polymer-bound catalyst and concentration of the filtrate. A further advantage conferred by the increased air-stability of the immobilised cobalt complexes is ease of handling using readily available laboratory equipment. Our results, together with the environmental advantages of immobilising metal carbonyls on a solid support, suggest that this new approach to the P–K reaction is worthy of considerable further investigation.
References
[1]Tetteh S. Imidazol-2-ylidene stabilized tetrahedral cobalt carbonyl complexes: A computational and structural database study. Heliyon. 2019 Jul 22;5(7):e02125. doi: 10.1016/j.heliyon.2019.e02125. PMID: 31372565; PMCID: PMC6658820.
[2]Comely, A. C., Gibson, S. E., & Hales, N. J. (2000). Polymer-supported cobalt carbonyl complexes as novel solid-phase catalysts of the Pauson-Khand reaction. *Chemical Communications*, 2000(4), 305-306. https://doi.org/10.1039/A909462H
- Related articles
- Related Qustion
- Reactions of Cobalt carbonyl Feb 24, 2022
Dicobalt octacarbonyl is a metal compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry.It is the pre
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCyclohexyl methacrylate forms MeCD complexes for aqueous polymerization; its polymer has low fragility, with hindered phenols altering Tg and relaxation.....
Oct 15,2025Chemical MaterialsCobalt carbonyl
10210-68-1You may like
- Cobalt carbonyl
-
- $35.00 / 1kg
- 2025-09-25
- CAS:10210-68-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Cobalt carbonyl
-

- $100.00 / 1kgkg
- 2025-06-20
- CAS:10210-68-1
- Min. Order: 1kgkg
- Purity: 99%
- Supply Ability: 100 tons
- Cobalt carbonyl
-
- $0.00 / 1KG
- 2024-08-06
- CAS:10210-68-1
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 1000KG