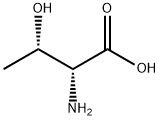
D-Threonine VS L-Threonine
Mar 28,2024
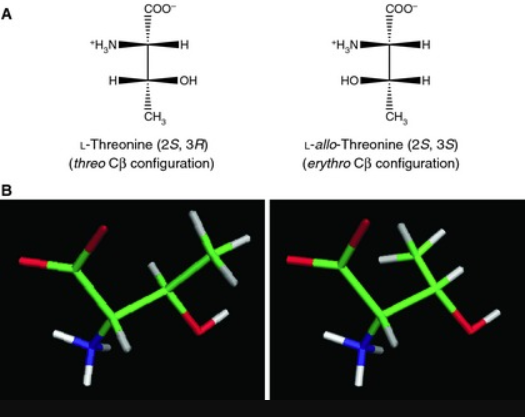
D-threonine is one of the essential unnatural amino acids and is used as a chiral building block of peptidomimetic drugs for the treatment of peripheral opioid side effects and analgesic applications. Threonine bears two chiral centers, leading to four stereochemical isomers, among which (2S, 3R) and (2R, 3S) configurations correspond to l- and d-enantiomers of threonine. In contrast to l-threonine, which is mass-produced by microbial fermentation for animal feed applications, fermentative production is not yet available for d-threonine. D-threonine is an optically active form of threonine having D-configuration. It has a role as a Saccharomyces cerevisiae metabolite. It is a threonine and a D-alpha-amino acid[2].
The stereochemical complexity of threonine renders stereoselective organic synthesis of d-threonine impracticable for an industrial scale-up due to low conversion and/or coproduction of stereochemical impurities. In contrast, the organic synthesis of dl-threonine with negligible diastereomeric impurities has been well established.
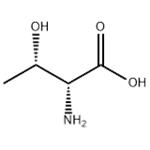
L-threonine is an essential amino acid; humans must obtain it from foods because their bodies cannot synthesize it. Foods that contain high amounts of protein, such as meats, fish, eggs, and dairy products, are good sources of L-threonine.
The L-Threonine tended to self-assemble with other L-Threonine molecules, while D-Threonine tended to self-assemble with other D-Threonine molecules. Both L-threonine and D-threonine formed triangle structures as well as chain structures. The chains and triangles differ in orientation between the two chiralities. Both L- and D- Threonine can each exist in two stereoisomers, which may explain the assembly of two distinct structures within each form (L- and D-). The self-assembled structures and orientation of LThreonine and D-Threonine may provide information on how stereoisomers interact; this could impact how amino acids are incorporated into proteins, potentially impacting protein structure and, therefore, function[2].
References
[1] Sang-Woo Han, Jong-Shik Shin. “Preparation of d-threonine by biocatalytic kinetic resolution.” Journal of Molecular Catalysis B-enzymatic 122 (2015): Pages 227-232.
[2 Midhun Ajikumar. "Threonine: An Amino Acid Autonomous Molecular Assembly."
- Related articles
- Related Qustion
- D-Threonine: An Important Unnatural Amino acids Oct 24, 2019
D-Threonine is one of the important unnatural amino acids and is used as a chiral building block of peptidomimetic drugs for treatment of peripheral opioids side effects and for analgesic applications.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringNilotinib Hydrochloride Monohydrate is the monohydrate monohydrochloride form of nilotinib, an orally bioavailable aminopyrimidine-derivative Bcr-Abl tyrosine kinase inhibitor with antineoplastic activity.....
Mar 28,2024APID-Threonine
632-20-2You may like
- Diosgenin:Uses,Functions and Synthesis
Dec 12, 2025
- Biosynthesis of Cyclopamine from Cholesterol
Dec 10, 2025
- Synthesis of ribociclib
Dec 10, 2025
- H-D-Thr-OH
-
- $0.00/ kg
- 2025-12-19
- CAS:632-20-2
- Min. Order: 1kg
- Purity: 98% 99%
- Supply Ability: 1T+
- D-Threonine
-

- $0.00 / 1Kg/Bag
- 2025-12-19
- CAS:632-20-2
- Min. Order: 1KG
- Purity: 98%-101%
- Supply Ability: 500KGS
- D-Threonine
-

- $999.00/ kg
- 2025-12-16
- CAS:632-20-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000