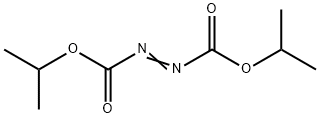
Diisopropyl azodicarboxylate: Application, synthesis and decomposition
May 10,2023
General description
Diisopropyl azodicarboxylate (DIAD) is the diisopropyl ester of azodicarboxylic acid. DIAD belongs to the azo compound and it can be used to initiate the polymerization reaction between the polymer monomers because it’s easy to decompose and form free radicals; meanwhile, because of this performance and exothermic reaction characteristics of Diisopropyl azodicarboxylate, this substance is very easy to decompose and release heat at a low temperature, resulting in self-accelerating decomposition, and finally leading to thermal runaway situation of the reaction. Recently, according to UN-GHS (Globally Harmonized System of Classification and Labelling of Chemicals), DIAD belongs to typical self-reactive substance, classified as the 8th item, which exists a risk of loss of control due to self-heating reaction during storage or transportation. Once, during production, transportation, application and storage, when shocked, rubbed, impacted or influenced by other external stimulation, Diisopropyl azodicarboxylate can trigger self-accelerating decomposition, resulting in fire or explosion accidents, and finally causing serious casualties and property losses. Its appearance is as follows:

Application
As an important intermediate in organic synthesis, Diisopropyl azodiformate (DIAD) is widely used in the field of polymer material synthesis [1]. It is used as a reagent in the production of many organic compounds. It is often used in the Mitsunobu reaction,[1] where it serves as an oxidizer of triphenylphosphine to triphenylphosphine oxide. It has also been used to generate aza-Baylis-Hillman adducts with acrylates.[2] It can also serve as a selective deprotectant of N-benzyl groups in the presence of other protecting groups.[3] It is sometimes preferred to diethyl azodicarboxylate (DEAD) because it is more hindered, and thus less likely to form hydrazide byproducts. One notable use of this compound is in the synthesis of Bifenazate.
Synthesis
Under nitrogen protection, 40g sodium methylate and 300ml anhydrous dimethyl sulphoxide is added in there-necked flask, slowly add 100g carbazic acid isopropyl ester and 100g diethyl carbonate successively, be warming up to 120 DEG C of reaction 3h, cooling, be the salt acid for adjusting pH value to 5 of 10% by solution massfraction, suction filtration, obtains hydrodiazo dioctyl phthalate diisopropyl ester.
At-20 DEG C ~ 25 DEG C, it is in the sulphuric acid soln of 40% that hydrodiazo dioctyl phthalate diisopropyl ester is added 300ml massfraction, after dissolving, slowly add 5g bromine, then control temperature of reaction at-15 DEG C ~-5 DEG C, drip the hydrogen peroxide extremely no longer heat release that massfraction is 10%, drip Bi Jixu and react 6h, use sodium bisulfite cancellation, with dichloromethane extraction, organic layer is washed to neutrality, anhydrous sodium sulfate drying organic layer, filter, concentrate and obtain diisopropyl azodiformate 79.1g, yield: 92.6%, purity: 98.9% [4].
Decomposition
Thermal decomposition behavior and decomposition mechanism of diisopropyl azodicarboxylate (DIAD) were characterized and analyzed by C80 micro-calorimetery and STA-FTIR-MS system [5]. The results show that only one exothermic peak appeared in decomposition process, and that the onset temperature and peak temperature increase with heating rate, whereas the released reaction heat at the heating rates remained approximately constant at 945.67 ± 23.45 kJ/kg. Based on C80 data, the calculated activation energy of diisopropyl azodicarboxylate decomposition ranges from 45 kJ/mol to 77 kJ/mol by Friedman method, while the reaction progress in the range of 0.10–0.90. During thermal decomposition process of DIAD, there is only one stage of weight loss, which starts at about 80 °C, reaches to the maximum rate of weight loss at 138 °C, and end up at about 150 °C. The thermal decomposition mechanism of diisopropyl azodicarboxylate can be mainly summarized as the route that the C-O single bond breaks at first, then C-C breaks, and the N=N bond breaks subsequently, then the C-O bond or the C-N bond breaks, final products such as H2O, CO2 etc. appear from further dissociation and oxidation of molecular ionic fragments at last.
References
[1]Zhang et al, Synthesis of diisopropyl azodicarboxylate, Chin. J. Appl. Chem. 32 (3) (2015) 261–266.
[2]Shi et al. (2004). Aza-Baylis–Hillman reactions of diisopropyl azodicarboxylate or diethyl azodicarboxylate with acrylates and acrylonitrile. Tetrahedron. 60 (9): 2083–2089.
[3]Kroutil et al. (2004). Improved procedure for the selective N-debenzylation of benzylamines by diisopropyl azodicarboxylate. Synthesis. 3 (3): 446–450.
[4]Li et al. Synthesis method of diisopropyl azodicarboxylate. China, CN104478758 A 2015-04-01.
[5]Jia et al. Thermal decomposition mechanism of diisopropyl azodicarboxylate and its thermal hazard assessment. Thermochimica Acta 688 (2020) 178601.
- Related articles
- Related Qustion
- Diisopropyl azodicarboxylate: self-accelerating decomposition and explosion Apr 28, 2024
DIAD belongs to the azo compound, and it can be used to initiate the polymerization reaction between the polymer monomers because it‘s easy to decompose and form free radicals
- What is Diisopropyl azodicarboxylate? Mar 3, 2021
Isopropyl azodicarboxylate (Acronym DIAD; foaming agent DIPA) belongs to diisopropyl azo-hydroxy acid salt. At room temperature, it is an orange-red transparent oily liquid with special smell and is soluble in almost all organic solvents.
Glyoxylic acid or oxoacetic acid is an organic compound that is both an aldehyde and a carboxylic acid.....
May 10,2023APIDiphenyl(2,4,6-trimethylbenzoyl)phosphine oxide is an organic photoinitiator. It is also an important organic intermediate to synthetize polymerization initiators in the area of materials science.....
May 10,2023SupplementsDiisopropyl azodicarboxylate
2446-83-5You may like
Diisopropyl azodicarboxylate manufacturers
- Diisopropyl azodicarboxylate
-

- 2025-12-14
- CAS:2446-83-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Diisopropyl azodicarboxylate
-

- $0.00 / 1KG
- 2025-12-13
- CAS:2446-83-5
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 500kgs/month
- Diisopropyl azodicarboxylate
-

- $0.00 / 1kg
- 2025-12-13
- CAS:2446-83-5
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise