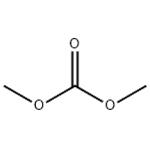
Dimethyl Carbonate: Production and Hazards
Nov 28,2022
Dimethyl carbonate (DMC) has been used as an intermediate to synthesize polycarbonate, polyurethane, medicine and agricultural chemicals. Dimethyl carbonate has an ester- or alcohol-like odor, which is more favorable to users than most hydrocarbon solvents it replaces.

Production
Dimethyl carbonate (DMC) is formed by the reaction of MeOH with phosgene or methyl chloroformate in the presence of a concentrated sodium hydroxide solution in a two-phase reaction in elevated yields and purity. Additional alcohols can also be phosgenated. As DMC is now more easily accessible via the direct oxidative carbonylation of MeOH, phosgenation is losing its attractiveness in this application.
Dimethyl carbonate is also manufactured by carbonylation of methylnitrite through a catalytic redox process. The process is practiced commercially in the gas phase by Ube Industries, LTD., Japan using a palladium supported catalyst system.
Hazards
Diethyl Carbonate can affect you when breathed in and may pass through the skin.
Contact can irritate the skin and eyes.
Breathing Diethyl Carbonate can irritate the nose and throat.
Exposure to Diethyl Carbonate can cause headaches, dizziness, nausea and vomiting, weakness and loss of consciousness.
Also, Diethyl Carbonate is a flammable liquid and a fire hazard.
- Related articles
- Related Qustion
- The wide range of applications: Dimethyl carbonate Apr 25, 2024
Dimethyl carbonate is a versatile compound that represents an attractive, eco-friendly alternative to both methyl halides (or dimethyl sulfate) and phosgene for methylation and carbonylation processes, respectively.
The passage describes how Potassium Phosphate works in food, and whether it is safe for us.....
Nov 28,2022APIHydroxypropyl - γ-cyclodextrin is a kind of compound with complexing ability.....
Nov 28,2022SupplementsDimethyl carbonate
616-38-6You may like
Dimethyl carbonate manufacturers
- Dimethyl Carbonate
-

- $1.00 / 1KG
- 2025-12-11
- CAS:616-38-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Dimethyl carbonate
-
- $0.00 / 200kg
- 2025-11-19
- CAS:616-38-6
- Min. Order: 20kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Dimethyl carbonate
-

- $0.00 / 5L
- 2025-08-22
- CAS:616-38-6
- Min. Order: 5L
- Purity: 99%
- Supply Ability: 20tons