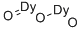Exploration of the Properties and Applications of Dysprosium Oxide
Apr 21,2025
Introduction
Dysprosium oxide (Dy2O3; Figure 1) is a rare earth metal oxide. It is highly insoluble and thermally stable. Dysprosium oxide reacts with acid to form dysprosium salt. Dysprosium fluoride (DyF3) can also be prepared by fluorinating dysprosium oxide with ammonium hydrogen fluoride reagent. Dysprosium metal can be prepared by thermal metal reduction of the resulting DyF3. Dysprosium oxide is widely used in ceramics, glass additives, high-efficiency phosphors, optical and electromagnetic materials. Dysprosium oxide nanoparticles are not only industrially usable, but also have promising biomedical applications as drug delivery candidates.

Exploratory study
1.The effect of compound addition Dysprosium oxide and Sn on the structure and properties of NdFeNbB magnets
NdFeNbB with the additions of Dysprosium oxide and Sn permanent magnets have been attained by means of powder-blending technique, and their magnetic properties, temperature performance and microstructure were studied in this paper. The addition of just 2.0wt% Dysprosium oxide or 0.3wt% Sn proved to be very effective in improving the permanent magnetic properties of NdFeNbB magnets. Dysprosium oxide additions result in the increase in the Hci and temperature dependence due to the increase of Tc, formation of (NdDy)-rich phase and grain refinement of Φ phase. This improvement of the coercivity stability of the magnets from the addition of Sn is attributed to the smoothing effect of the Sn addition at the grain boundaries. The magnetic properties, the temperature dependence and Curie temperature of NdFeNbB with Dysprosium oxide and Sn combined addition were found to be considerably improved. From the X-ray diffraction, SEM-EDAX studies and the thermo-magnetic study, the improved properties due to the solution of Dy and Sn to the Φ phase, the reduced Neff and the smaller Φ phase.
2.Structural properties and electrical characteristics of high-k Dysprosium oxide gate dielectrics
This paper describes the structural properties and electrical characteristics of thin dysprosium oxide dielectrics deposited on silicon substrates by means of reactive sputtering. The structural and morphological features of these films after postdeposition annealing were studied by X-ray diffraction and X-ray photoelectron spectroscopy.It is found that dysprosium oxide dielectrics annealed at 700 ℃ exhibit a thinner capacitance equivalent thickness and better electrical properties, including the interface trap density and the hysteresis in the capacitance–voltage curves. Under constant current stress,the Weibull slope of the charge-to-breakdown of the 700 ℃-annealed films is about 1.6. These results are attributed to the formation of well-crystallized dysprosium oxide structure and the reduction of the interfacial SiO2 layer.
3.Homogeneous Precipitation of Dysprosium oxide Nanoparticles-Effects of Synthesis Parameters
In this paper, homogeneous precipitation technique using urea is reported to prepare dysprosium oxide nanoparticles at low processing temperature and high yield.The as-precipitated particles had an amorphous microstructure, which was converted into dysprosium oxide by heating in air at 700℃ for 2 hours.The aggregation mechanism has been offered to explain the growth of the nanoparticles by urea precipitation, where the growth of the particle is influenced by stability of secondary particles and collisions among the primary particles.The stability of the secondary particles can be related to the electrical double thickness surrounding the particles, where the thickness is affected by the concentration of ions present in the solution. Dysprosium ion concentration was found to have a significant effect on the size and size distribution of the nanoparticles, while urea concentration and aging time had less significance. In addition, chlorine ion was found to promote aggregation among the particles since it reduces the electrical double thickness.[3]
4. Experimental and theoretical approach on the optical properties of zinc borotellurite glass doped with dysprosium oxide.
A series of glass samples with chemical formula {[(TeO2)0.7(B2O3)0.3]0.7(ZnO)0.3}1-x(Dy2O3)x where x=0.01, 0.02, 0.03, 0.04 and 0.05M fraction were synthesized through conventional melt-quenching method. The most common way to fabricate a glass material is by fusion of two or more component oxides followed by their quenching. This technique is known as melt-quenching technique. Kaur et al. [4] highlighted that the melt-quenching method able to enhance the mechanical properties like hardness and flexural strength of the material. The nature of the glass systems is proven to be amorphous based on the XRD pattern. The FTIR spectra of the glass systems confirm the existence of five bands which are assigned for the BO4, BO3, TeO4 and TeO3 vibrational groups. The density of the glass systems is increased with the addition of dysprosium oxide while the molar volume is found to be inversely proportional to the density of the proposed glass. The optical properties of the glasses are determined through the absorption spectra obtained from the UV-VIS spectrophotometer. From the absorption spectra, the indirect and direct optical band gaps and the Urbach energy are found to be inversely proportional to each other. As the molar fraction of the dysprosium oxide increased, the optical band gaps are observed to increase as opposed to the Urbach energy. For this glass system, the values of refractive index, electronic polarizability, oxide ion polarizability and the optical basicity are found to decrease as the addition of the dysprosium oxide is increased. From the emission spectra, two intense blue and yellow emission bands are observed, which correspond to the 4F9/2→6H15/2 and 4F9/2→6H13/2 transitions of Dy3+ ions respectively. The CIE chromaticity coordinates of the zinc borotellurite glass systems are found to be located in the white light region.The CIE coordinateof the glass systems are closed to the coordinate for the standardwhite light thus suggest that the zinc borotellurite glass doped with dysprosium oxide may be apply in the solid state lighting application.[5]
References
[1] Li L , Yi J , Peng Y ,et al.The effect of compound addition Dy2O3 and Sn on the structure and properties of NdFeNbB magnets[J].Journal of Magnetism & Magnetic Materials, 2007, 308(1):80-84.DOI:10.1016/j.jmmm.2006.05.005.
[2] Pan T M , Chang W T , Chiu F C .Structural properties and electrical characteristics of high-k Dy2O3 gate dielectrics[J].Applied Surface Science, 2011, 257(9):3964-3968.DOI:10.1016/j.apsusc.2010.11.144.
[3] Tok A I Y , Su L T , Boey F Y C ,et al.Homogeneous Precipitation of Dy2O3 Nanoparticles—Effects of Synthesis Parameters[J].Journal of Nanoscience and Nanotechnology, 2007, 7(3):907-915.DOI:10.1166/jnn.2007.203.
[4] Kaur G, Pickrell G, Sriranganathan N, Kumar V, Homa D. Review and the state of the art: Sol-gel and melt quenched bioactive glasses for tissue engineering. J Biomed Mater Res B Appl Biomater. 2016;104(6):1248-1275. doi:10.1002/jbm.b.33443
[5] Halimah MK, Ami Hazlin MN, Muhammad FD. Experimental and theoretical approach on the optical properties of zinc borotellurite glass doped with dysprosium oxide. Spectrochim Acta A Mol Biomol Spectrosc. 2018;195:128-135. doi:10.1016/j.saa.2017.12.054
- Related articles
- Related Qustion
- Crystal Structure of Dysprosium oxide Jun 13, 2024
Dysprosium oxide (Dy2O3) is a rare earth metal oxide. It is highly insoluble and thermally stable. Dy2O3 reacts with acid to form dysprosium salt.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringTulathromycin is a semi-synthetic macrolide antibiotic that is highly effective in treating respiratory tract bacterial infections.....
Apr 21,2025DrugsDysprosium oxide
1308-87-8You may like
Dysprosium oxide manufacturers
- Dysprosium Oxide
-

- $1.00 / 1kg
- 2025-12-11
- CAS:1308-87-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Dysprosium Oxide
-

- $230.00 / 1kg
- 2025-11-21
- CAS:1308-87-8
- Min. Order: 1000kg
- Purity: 99%
- Supply Ability: 20T per moth
- Dysprosium oxide
-

- $6.00 / 1kg
- 2025-07-29
- CAS:1308-87-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month