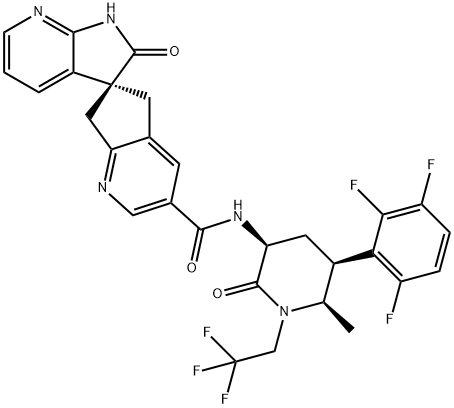
How is Atogepant synthesised?
Jan 15,2024
Synthesis of Atogepant
Atogepant was synthesised from the late-stage amide coupling of two highly complex fragments (Carboxylic Acid Fragment and Spirocyclic Motif Fragment). The specific synthesis steps are as follows:
Step 1: Preparation of Carboxylic Acid Fragment for Synthesis of Atogepant
The synthesis of the carboxylic acid fragment began with diacid 10.1, which was converted to the bis-ester under acidic conditions, followed by bromination to afford bromopyridine 10.2. Reduction of both esters and subsequent mesylation gave 10.3. Treatment of mesylate 10.3 with pyridone 10.4 under basic conditions afforded racemic spirocycle 10.5. Palladium-mediated carbonylation resulted in the methyl ester, which was purified by using chiral supercritical fluid chromatography (SFC) to afford a single enantiomer of pyridine 10.6. Ester hydrolysis and SEM cleavage gave the desired carboxylic acid fragment 10.7. No yields were reported for any step in this sequence.

Step 2: Asymmetric Synthesis of Related Spirocyclic Motif
The route to 10.7 as described utilized chiral SFC to isolate the desired spirocyclic enantiomer, which is not a common purification method on scale. Other asymmetric routesto similar fragments have also been described, one of which is shown in step 1. Racemic alkyl chloride 10.8 was cyclized under aqueous conditions to give spirocycle 10.9 using quinidine derivative 10.10. After crystallization, the spirocycle was isolated in 72% yield with 99% ee. Catalyst 10.10 was prepared in a single step from commercially available quinidine.

Step 3: Preparation of Atogepant
To synthesize the amine fragment of the atogepant, carboxylic acid 10.11 was converted to the corresponding Weinreb amide 10.12 in 99% yield via an acid chloride generated using POCl3. Conversion of the Weinreb amide to the methyl ketone proceeded smoothly in 95% yield. Enolate formation from ketone 10.13 was accomplished using LiOt-Bu and ZnBr2. Treatment with racemic mesylate 10.14 afforded amino acid derivative 10.15 in 67% yield. Enzymatic reductive amination of ketone 10.15 with a transaminase induced cyclization to pyridone 10.16 in 71% yield, with a >60:1 cis:transdiastereomeric ratio at C5:C6 and a 1:1 mixture of epimers at C3. Epimerization of 10.16 using t-BuOK afforded pyridone 10.17 in an 85% yield.
Selective N-alkylation using triflate 10.18 resulted in pyridone 10.19 in 92% yield as a 6.5:1 cis:transmixture of diastereomers at the C3 position. The primary amine at C3 was revealed via an acidic deprotection, and then treatment with amino acid 10.20 gave the salt 10.21 in 89% yield. To complete the synthesis, amine 10.21 was free-based and subjected to an EDC coupling with carboxylic acid 10.7 to afford atogepant in 95% yield.

- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPterostilbene exhibits increased bioavailability due to the presence of two methoxy groups, which cause it to exhibit increased lipophilic and oral absorption.....
Jan 15,2024Biochemical EngineeringAtogepant (MK-8031)
1374248-81-3You may like
Atogepant (MK-8031) manufacturers
- Atogepant
-
- $158.00 / 1mg
- 2025-12-10
- CAS:1374248-81-3
- Min. Order:
- Purity: 99.66%
- Supply Ability: 10g
- Atogepant (MK-8031)
-

- $0.00 / 25kg
- 2025-12-01
- CAS:1374248-81-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000kg
- Atogepant (MK-8031)
-

- $0.00 / 25KG
- 2025-12-01
- CAS:1374248-81-3
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 10000KGS