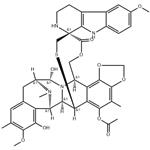
How is Lurbinectedin synthesised?
Jan 2,2024
Synthesis of Lurbinectedin
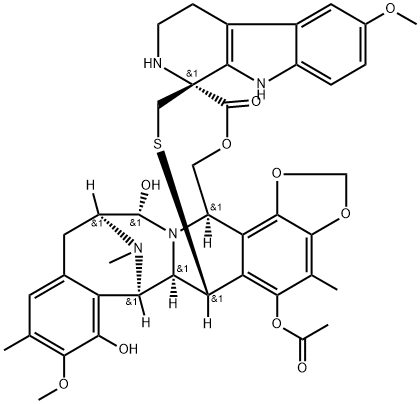
Lurbinectedin is synthesised using tyrosine derivative as the raw material, firstly the intermediate Lurbinectedin Bridged Azocane is synthesised, and then through esterification and cyclisation. The specific synthesis steps are as follows:
Step 1: Preparation of Lurbinectedin Bridged Azocane
Tyrosine derivative 260 underwent a PictetSpengler reaction with aldehyde 261 followed by N-Boc protection to furnish tetrahydroisoquinoline 262 in excellent yield. Oxidative dearomatization using salcomine and cobalt salen catalyst 263 gave an intermediate quinone, which underwent blue light-facilitated benzo[1,3]dioxole formation and rearomatization through a light-mediated remote C−H bond activation to generate 264 in 71% over two steps. Phenol protection followed by Swern oxidation proceeded smoothly to afford aldehyde 265 in 87% yield for two steps. Next, another Pictet-Spengler reaction between aldehyde 265 and amine 260 produced pentacycle 266 in 67% yield. This reaction was followed by N-methylation of the secondary amine under modified Eschweiler-Clarke conditions and allylation of the phenol to arrive at allyloxy ether 267. Oxidation of the primary alcohol to the aldehyde, followed by an intramolecular Strecker reaction and subsequent deprotection of both benzyl protecting groups using boron trichloride, furnished bridged azocane 268 in 79% yield for three steps.
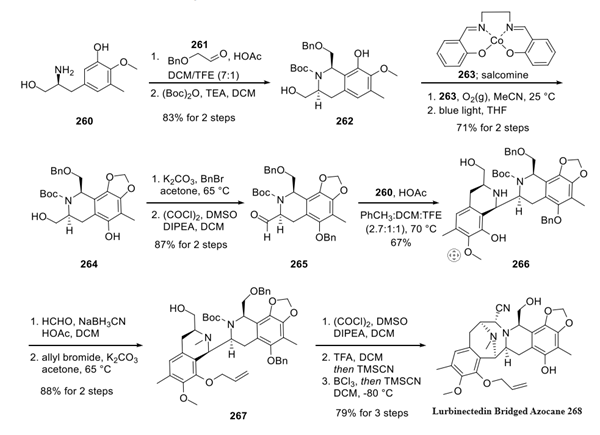
Step 2: Preparation of Lurbinectedin
Oxidative dearomatization of the phenol with benzeneselenic anhydride, followed by esterification of the primary alcohol with (R)-N-Alloc-S-Fm-Cys (269), generated hydroxy enone 270 in 72% over two steps. Macrocyclization was accomplished following Corey's reported one-pot procedure, which afforded sulfide 271 in 51%yield. Reductive removal of both the allyl and alloc protecting groups produced amine 272 in 85% yield, after which the reaction with 4-formyl-1-methylpyridinium benzenesulfonate (273) gave oxalate ester 274 in 52% yield. Finally, a Pictet-Spengler reaction with tryptamine derivative 275 followed by iminium-mediated ejection of the nitrile moiety and hydrolytic quench afforded lurbinectedin in 77% yield over two steps.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPemigatinib is synthesised using pyrrolopyridine as a raw material, firstly by synthesis of Pemigatinib Morpholine Intermediate and then by reduction, cyclisation and other reactions.....
Jan 2,2024InhibitorsLurbinectedin
497871-47-3You may like
Lurbinectedin manufacturers
- Lurbinectedin
-

- $1930.00 / 5mg
- 2025-11-04
- CAS:497871-47-3
- Min. Order:
- Purity: 98.11%
- Supply Ability: 10g
- Lurbinectedin
-
- $6.00 / 1KG
- 2025-09-25
- CAS:497871-47-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Lurbinectedin
-

- $0.00 / 1g
- 2025-09-11
- CAS:497871-47-3
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month