How to synthesize Plitidepsin?
Jan 3,2024
Description
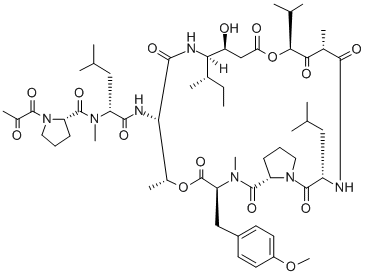
Plitidepsin, dehydrodidemnin B, is a macrocyclic marine natural product isolated nearly 40 years ago from the tunicate aplidium albicans. The drug showed potent antiproliferative effects in cancer cells and in vivo tumor models in early studies. While plitidepsin has demonstrated a broad range of pleiotropic effects in cells, interaction with the translation elongation factor eEF1A2 has been proposed as the primary mechanism of action.
Synthesis method
The synthetic route to plitidepsin reported on the largest scale is shown below[1]. This route employed a convergent approach whereby the construction of two main fragments yielded a linear precursor, which then underwent macrolactamization prior to the final introduction of peptidic side chains.
Assembly of Plitidepsin Peptidic Fragment 326

The synthesis of the first fragment commenced with the protected bis-peptide 321, which was treated with HCl in dioxane to remove the Boc group, affording 322 (Scheme 1). Next, reductive debenzylation of keto ester 323 made way for direct coupling with 322 under standard amide forming conditions to yield 324. Fluoride-mediated TBS removal was followed by esterification with acid 325, and simultaneous cleavage of Boc and silyl protecting groups with HCl generated 326.
Preparation of Plitidepsin Ketoester Fragment 323

The synthesis of 323 started with bis-silylation of 327 followed by selective silyl ester hydrolysis to provide 328 in high yield. Activation of 328 with CDI formed an acyl imidazole species that underwent treatment with the lithium enolate of benzyl acetate to afford keto ester 329. Deprotonation followed by exposure to iodomethane generated malonate 323 as a mixture of diastereomers, as shown above.
Preparation of Plitidepsin Carboxylic Acid 325
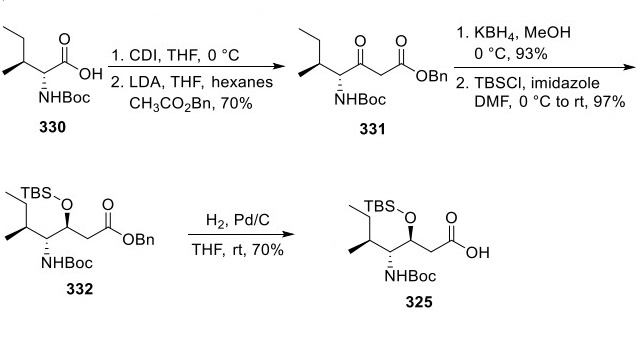
The preparation of 325 started with converting N-Boc isoleucine (330) to keto ester 331 (Scheme 3). KBH 4-mediated reduction of the ketone within 331 was completed in high yield and diastereoselectivity, and silylation of the resultant alcohol yielded 332. Deprotection of the ester was accomplished with catalytic hydrogenation to give 325 in good yield.
Construction of Plitidepsin Peptide Fragment 338
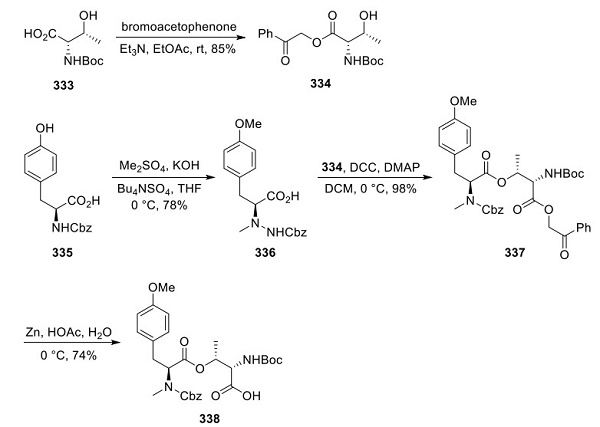
Synthesis of 338, the second fragment necessary for coupling with 326, started with protected threonine derivative 333 (Scheme 4). Intermediate 333 was esterified via S N 2 reaction of the carboxylate with bromoacetophenone, yielding 334. The protected tyrosine intermediate 335 was N-alkylated under phase transfer conditions to give phenethylamine 336, which was then esterified with 334 to afford 337 in high yield. Selective removal of the phenacyl ester was made possible upon zinc and acetic acid exposure, generating 338 in 74% yield.
Final Assembly of Plitidepsin
The macrocyclization precursor was prepared by coupling 326 and 338 using well-established amide bond-forming methods to afford 339 (Scheme 5). Hydrogenation using Pearlman’s catalyst proceeded in high yield, revealing the acid and secondary amine groups used to form macrocycle 340. The ring-closing reaction was performed by treatment with HATU, HOAt, and NMM under high dilution, resulting in macrocycle 340 in 68% yield and forming the core structure of plitidepsin. Notably, the β-keto ester stereocenter carried forward as a mixture of diastereomers in the linear precursor, was set during the macrocyclization process to yield a single stereoisomer. A high-yielding, three-step process to convert 340 to 343 consisted of Boc removal under acidic conditions to liberate the primary amine, coupling of Cbz-protected N-Me amino acid 342 (formed by selective N-methylation of 341 using NaH and MeI in THF) to the primary amine, and finally, hydrogenolytic cleavage of the Cbz group. Coupling of the proline-derived acid 343 to 344 using diisopropyl carbodiimide (DIC) completed the synthesis of plitidepsin.
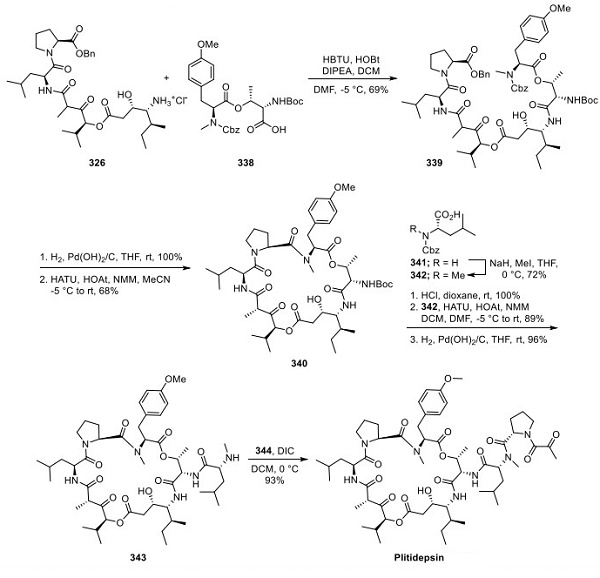
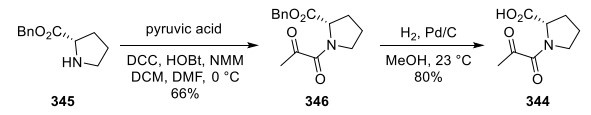
To generate 344, proline benzyl ester 345 was coupled with pyruvic acid to yield 346, followed by conversion of the benzyl ester to the acid via hydrogenation (Scheme 6).
References
[1] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
- Related articles
- Related Qustion
- Pharmacological activity of Plitidepsin Jul 1, 2022
Plitidepsin (also known as dehydrodidemnin B, marketed by PharmaMar, S.A. under the trade name Aplidin) is a chemical compound extracted from the ascidian Aplidium albicans.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering4-Butylresorcinol 0.1% Cream has shown rapid efficacy and good tolerability when used in the treatment of melasma.....
Jan 3,2024APIPlitidepsin
137219-37-5You may like
- Diosgenin:Uses,Functions and Synthesis
Dec 12, 2025
- Biosynthesis of Cyclopamine from Cholesterol
Dec 10, 2025
- Synthesis of ribociclib
Dec 10, 2025
- Plitidepsin
-
- $8.00 / 1kg
- 2025-09-25
- CAS:137219-37-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Plitidepsin
-
- $998.00 / 1mg
- 2021-05-08
- CAS:137219-37-5
- Min. Order: 1mg
- Purity: 97%
- Supply Ability: 10mg