Is sodium a metal or nonmetal?
Mar 8,2024
Sodium is a metal. Firstly, from the periodic table we can see that sodium (Na) has an atomic number of 11 and is a member of the alkali metal family.
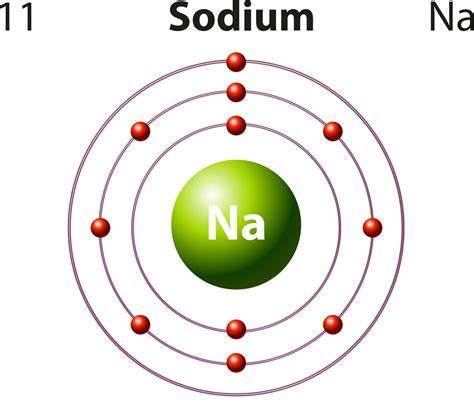
Secondly, according to the definition of a metal, a metal is an element that loses electrons easily and is lustrous and ductile, and is a good conductor of heat and electricity. Sodium is a member of group 1 and therefore loses electrons easily. It has a silvery-white, lustrous appearance; it is ductile at room temperature and can be easily cut or moulded into shapes with a butter knife without breaking.
Sodium is a good conductor of electricity. This is because the potential difference allows free electrons to move through the metal thus conducting electricity. Sodium is also a good conductor of heat; in the metallic structure of sodium, the cations are located close to each other with a symmetrical geometrical layout. As the ions maintain their position in the lattice, their vibrations are constant. Heating the metal causes these ions to "collide" with other ions, causing them to vibrate as well. Transferring energy in this way is how heat is transferred quickly from one end to the other. Sodium is therefore a good conductor of heat.
Finally, sodium has a relatively high melting and boiling point and is highly reactive compared to most non-metals. Therefore, sodium is a metal.
- Related articles
- Related Qustion
- How many neutrons, protons and electrons does a sodium atom have? Mar 6, 2024
A neutron is a neutral subatomic particle that, in conjunction with protons, makes up the nucleus of every atom except ordinary hydrogen (whose nucleus has one proton and no neutrons).
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringD-(-)-Salicin, a phenolic glycoside, undergoes enzymatic biosynthesis and metabolism into salicylic acid, highlighting its significant anti-inflammatory and pro-apoptotic medicinal potential.....
Dec 10,2024Biochemical EngineeringSodium
7440-23-5You may like