Maraviroc: mechanism, pharmacology, metabolism and its potential application
May 12,2025
Introduction
Maraviroc, first in a new pharmacological class of antiretroviral agents known as CCR5 antagonists, has been approved by the U.S. Food and Drug Administration for the treatment of HIV-infected adult patients who are infected with only CCR5-tropic HIV-1 virus and who have HIV-1 strains resistant to multiple antiretroviral agents.(Figure 1) [1] Maraviroc has demonstrated in vitro activity against a wide range of CCR5 tropic clinical isolates, including those resistant to the four currently existing drug classes of antiretroviral agents. In the two pivotal phase III studies, MOTIVATE-1 and -2, maraviroc,in combination with optimized background therapy(OBT), demonstrated superior virologic and immunologic treatment outcomes than OBT alone intreatment-experienced patients infected with CCR5-tropic HIV-1. In these studies, maraviroc also demonstrated acceptable safety and tolerability profiles with adverse events and discontinuation rates in general comparable to those noted in the placebo arms.[2]

Structure and mechanism of action
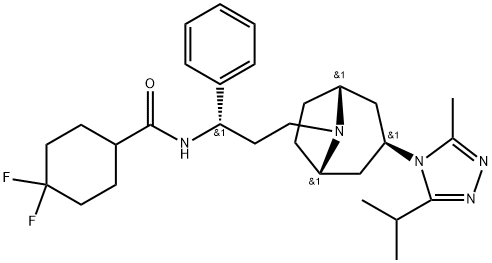
Maraviroc is a selective, reversible, noncompetitive, functional antagonist of CCR5 receptor. Its chemical name is 4,4-Difluoro-N-[3-[(1R,3exo,5S)-3-(3-isopropyl-5- methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo- [3.2.1]oct-8-yl]-1(S)-phenyl-propyl]cyclohexane carboxamide; its empirical formula is C29H41F2N5O.
Maraviroc allosterically binds to CCR5 and induces conformational changes within CCR5 that result in the inhibition of its binding to gp120, thus preventing CCR5-mediated virus–cell fusion and subsequent cell entry of M-tropic HIV-1. It has no efficacy against T-tropic or dual-tropic virus. On a molecular level, maraviroc inhibits chemokine-dependent activation of GDP–GTP exchange and chemokine-induced intracellular calcium redistribution thus displaying both binding and signaling blockade. The IC50 for inhibition of gp120 binding to CCR5 was 11 nM. Maraviroc has also exhibited a prolonged CCR5 physical and functional occupancy.[2]
Pharmacology and metabolism
Pharmacokinetic and metabolic studies were conducted in mice, rats, dogs and humans. After a single intravenous administration in rats and dogs, half-life values of 0.9 and 2.3 hours, respectively, were observed with a large volume of distribution in both species (6.5 l/kg and 4.3 l/kg,respectively). Plasma protein binding was moderate: 58% in mice, 51% in rats and 63.7% in dogs.Oral bioavailability in rats and dogs was 6% and 40%, respectively.In humans, oral administration of maraviroc had a nonlinear pharmacokinetic pattern where a 10-fold increase in dose resulted in a 40-fold increase in maximum plasma concentration (Cmax)and a 20-fold increase in the area under the curve(AUC) accompanied by an increase in the rate of absorption. Protein binding in human plasma is reported to be 75.5%. Maraviroc did not show any significant activity when evaluated in a number of human in vitro immune function assays using doses that exceeded 1,000 times the IC50 for maraviroc. Similarly, no significant activity against a range of pharmacologically relevant enzymes, ionchannels and receptors was noted at concentrations of maraviroc up to 10 µM. In a phase I single- and multiple-dose pharmacokinetic study conducted on healthy male volunteers for 12 days, maraviroc showed rapid absorption and achieved Cmax within 0.5-4.0 hours following dosing. Maraviroc is a substrate for thecytochrome P450 3A4 isoenzyme (CYP3A4) as well as for p-glycoprotein. In addition to the CYP3A4 pathway, maravirocis also metabolized through oxidative mechanisms. The major route of excretion following oraladministration is fecal in all species (72-94% in animals; 76.4% in humans). The level of unchanged drug in urine in humans is 8% of administered drug.[2]
Special Populations
No studies were identified that examined the pharmacokinetics of maraviroc in specific patient populations. Analyses of pooled Phase I/IIa population pharmacokinetic data revealed no clinically significant differences in the disposition of maraviroc with regard to sex or race. Therefore, dose adjustment on the basis of sex or ethnicity does not appear to be necessary.[1]
Potential application of maraviroc in the therapy of neuropathic pain
According to International Association for the Study of Pain (IASP) neuropathic pain is defined as a pain caused by a lesion or disease of the somatosensory nervous system. In general population 7-8% adults suffer from chronic pain with neuropathic characteristic. The most common causes include: lumbar radiculopathy, postherpetic neuropathy, HIV infection, autoimmune diseases (multiple sclerosis), metabolic diseases (diabetic neuropathy), stroke or spinal cord injury. Current pharmacotherapy of neuropathic pain has insufficient effectiveness, so comprehension of neuropathic pain mechanism is necessary for research of new therapeutic methods. In the study we verify the analgesic effect of maraviroc (antagonist of the chemokine receptor - CCR5) and its potential role in the treatment of neuropathic pain. In the study, Sojka P etal. focused on dependency between opioid and chemokine receptors, because of similar structure between this receptors occurs cross-desensitization phenomenon. Chemokine antagonist maraviroc belongs to a group of entry inhibitors, antiretroviral drug. It enhances analgesic properties of opioids by inhibition of cross-desensitization of opioid's receptor. Application of maraviroc with morphine can reduce effective dosage of morphine 2,3 fold. Moreover, research show that prophylactic administration of maraviroc without opioid analgesics suppresses development of neuropathic pain symptoms. It has influence on glial phenotype, decreases secretion of proinflammatory cytokines and increases anti-inflammatory cytokine secretion. Furthermore it decreases expression of chemikine receptor mRNA and chemikine ligand's secreted by microglia and astrocytes as a result of nerve injury. They conclude that maraviroc has immunomodulatory properties, potentiates opioid analgesics effect, and can be used in neuropathic pain therapy as a potential co-analgesic.[3]
References
[1]Lieberman-Blum SS, Fung HB, Bandres JC. Maraviroc: a CCR5-receptor antagonist for the treatment of HIV-1 infection. Clin Ther. 2008;30(7):1228-1250. doi:10.1016/s0149-2918(08)80048-3
[2]Fadel H, Temesgen Z. Maraviroc. Drugs Today (Barc). 2007;43(11):749-758. doi:10.1358/dot.2007.43.11.1131763
[3]Sojka P, W?aszczuk A, Olakowska E. Potencjalne zastosowanie marawiroku w terapii bólu neuropatycznego. Potential application of maraviroc in the therapy of neuropathic pain. Pol Merkur Lekarski. 2021;49(293):379-381.
- Related articles
- Related Qustion
- Side effects of Maraviroc Apr 2, 2022
Maraviroc was synthesized by Pfizer Inc as UK-427,857 (4, 4-difluoro- N-[(1S)-3-[exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[ 3.2.1]oct-8-yl]-1-phenylpropyl]cyclohexanecarboxamide). The molecular weight of maraviroc is 5
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringSchizandrol A is an active component in schisandra with some pharmacological activities, also the representative component for the identification of schisandra.....
May 12,2025Chinese HerbsMaraviroc
376348-65-1You may like
- Maraviroc
-
- $0.00 / 1kg
- 2025-12-17
- CAS:376348-65-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Maraviroc
-

- $10.00 / 1KG
- 2025-12-11
- CAS:376348-65-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Maraviroc
-

- $0.00 / 1Kg/Bag
- 2025-11-19
- CAS:376348-65-1
- Min. Order: 0.1Kg/Bag
- Purity: 96% min, GMP,Food grade; Medicine grade
- Supply Ability: 20 tons