Side effects of Maraviroc
Apr 2,2022
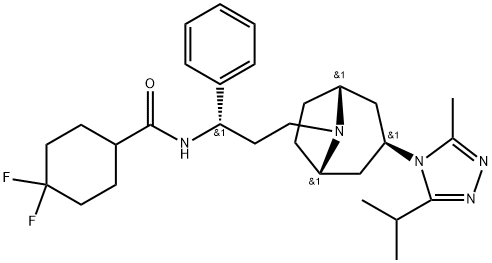
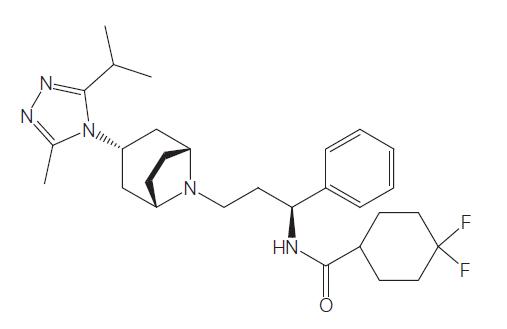
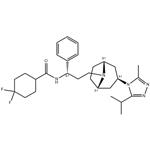
Maraviroc was synthesized by Pfizer Inc as UK-427,857 (4, 4-difluoro- N-[(1S)-3-[exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[ 3.2.1]oct-8-yl]-1-phenylpropyl]cyclohexanecarboxamide). The molecular weight of maraviroc is 514 and its chemical structure is shown below. Maraviroc is approved by the US FDA for use in treatmentexperienced individuals infected with CCR5-tropic HIV-1 strains. Maraviroc is marketed as Celsentris or Selzentrys and belongs to a very heterogenous class of HIV-1 inhibitors which prevent entry of virus into cells, including enfuvirtide. Maraviroc prevents HIV-1 gp120 envelope glycoproteins from binding to the CCR5 HIV-1 coreceptor, thus preventing entry of CCR5-using (so-called R5) HIV-1 strains. Maraviroc has no activity against CXCR4-using (X4) HIV-1 strains, and has no activity toward HIV-2 or other viruses.
Uses
As maraviroc is only active against HIV-1 strains which enter cells via the CCR5 chemokine receptor (R5 strains), laboratory testing to assess the tropism of the patient’s HIV-1 strain is essential before treatment with maraviroc. The most commonly used test to determine HIV-1 tropism is the Trofilet assay developed and marketed by Monogram Biosciences Inc. (San Francisco, CA, USA), which is based on the ability of laboratory strains of HIV-1 which are engineered to express the envelope glycoprotein of the patient’s virus to infect cells expressing CD4 and either CCR5 or CXCR4.
Currently, the Trofile assay can detect X4-tropic variants that are 5–10% of the total plasma viral quasispecies, but recent improvements to the assay have been reported to detect 0.5–1% of X4-tropic variants. Analysis of viral populations in antiretroviral-experienced subjects receiving CCR5 antagonists as monotherapy or as components of combination therapy, who experienced emergence of X4- or dual-tropic (using R5 and X4 coreceptors) virus, suggest these strains were pre-existing and not the result of virus evolution owing to the presence of the CCR5 inhibitor. Thus, the presence of any detectable X4- or dual-tropic virus before initiation of maraviroc is a critical predictor of maraviroc failure, and is a contraindication to maraviroc therapy.
Mechanism of action
HIV-1 entry occurs via sequential interaction of the gp120 envelope glycoproteins, studding the exterior of the HIV-1 particle, first with the primary receptor, CD4, and then with a coreceptor. Engagement of gp120 with the coreceptor triggers fusion of virion and cell membranes via the gp41 fusion peptide. The principal coreceptors used by HIV-1 for entry in vivo are the 7-transmembrane domain chemokine receptors CCR5 or CXCR4. Maraviroc is a noncompetitive allosteric antagonist of CCR5. Maraviroc binds CCR5, but not the closely related CCR2 chemokine or CXCR4 receptors. Therefore, maraviroc blocks the entry of R5-tropic HIV-1 strains into cells, but not X4-tropic strains. Maraviroc binds CCR5 reversibly and does not induce receptor internalization, and as maraviroc does not compete with the binding of chemokines to CCR5, it should not affect cellular signaling via CCR5.
Bioavailability
Maraviroc is not well absorbed when given by mouth. Bioavailability varied considerably with dose, with a bioavailability of 23% for a single 100-mg dose increasing to a bioavailability of 33% with a 300-mg dose. In studies of single 300-mg oral doses of radiolabeled maraviroc in volunteers, within a 168-hour period, 76% of both metabolized and unmetabolized forms of the drug were excreted in feces, with a further 20% found in urine: a total of 96%. Although a large proportion (25%) of a single 300-mg oral dose of maraviroc was excreted unchanged (i.e. unmetabolized) in feces, and this could be drug passing through the gastrointestinal tract unchanged, intravenous administration of maraviroc to animals showed secretion of unchanged maraviroc in bile and active secretion across the gut wall.
Maraviroc is moderately bound to plasma protein in humans with a mean value of 76%. The mean blood–plasma partitioning ratio was 0.59, with a range from 0.51 to 0.65. The volume distribution of maraviroc was approximately 2.8 l/kg (196 liters) with a clearance rate of 10.5 ml/min per kg.
Side effects
Maraviroc can cause serious, life-threatening side effects. These include liver problems, skin reactions, and allergic reactions. An allergic reaction may happen before liver problems occur.Official labeling of Selzentry has black box warning for hepatotoxicity.The MOTIVATE trials showed no clinically relevant differences in safety between the maraviroc and placebo groups.
- Related articles
- Related Qustion
- Maraviroc: mechanism, pharmacology, metabolism and its potential application May 12, 2025
Maraviroc represents the first licensed CCR5 co-receptor antagonist from the new antiretroviral (ARV) drug-class of entry inhibitors.
Enfuvirtide is an antiretroviral drug used in salvage regimens in (usually) highly drug-experienced individuals that suppresses replication of HIV-1 strains with multidrug resistance to reverse transcriptase inhibitors and protease inhibito....
Apr 2,2022APIElvitegravir (brand name Vitekta) is a drug used as part of antiretroviral therapy (ART). The FDA approved elvitegravir in 2012 as an antiretroviral drug (ARV) for people with HIV infection. Elvitegravir is manufactured by Gilead Sciences.....
Apr 2,2022APIMaraviroc
376348-65-1You may like
- Maraviroc
-
- $0.00 / 1kg
- 2025-12-13
- CAS:376348-65-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Maraviroc
-

- $10.00 / 1KG
- 2025-12-11
- CAS:376348-65-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Maraviroc
-

- $0.00 / 1Kg/Bag
- 2025-11-19
- CAS:376348-65-1
- Min. Order: 0.1Kg/Bag
- Purity: 96% min, GMP,Food grade; Medicine grade
- Supply Ability: 20 tons