Poly(1-vinylpyrrolidone-co-vinyl acetate): HME Mixing, Peroxide Control & MA Dispersions
Oct 21,2025
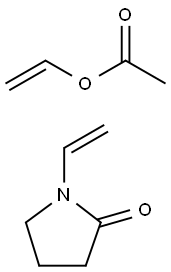
Poly(1-vinylpyrrolidone-co-vinyl acetate) can be used as a starting material to prepare a quasi-solid polymer electrolyte (QSPE) for dye-sensitized solar cells. It can be used to preparepolymer blend membranes for pervaporation separation of ethanol and2-ethylhexanol mixtures. Poly(1-vinylpyrrolidone-co-vinyl acetate) is used as a tablet binder, a film-former, and as part of the matrix material used in controlled-release formulations. In tableting, copovidone can be used as a binder for direct compression and as a binder in wet granulation. Copovidone is often added to coating solutions as a film-forming agent. It provides good adhesion, elasticity, and hardness, and can be used as a moisture barrier. Poly(1-vinylpyrrolidone-co-vinyl acetate) is used widely in pharmaceutical formulations and is generally regarded as nontoxic. However, it is moderately toxic by ingestion, producing gastric disturbances. It has no irritating or sensitizing effects on the skin. A study was conducted to look at the carcinogenicity and chronic toxicity of copovidone (Kollidon VA 64) in Wistar rats and Beagle dogs. The results of these studies demonstrated the absence of any significant toxicological findings of high dietary levels of copodivone in rats and dogs, resulting in noobserved- adverse-effect levels of 2800 mg/kg body-weight/day in rats and 2500 mg/kg body-weight/day in dogs, the highest doses tested.

Assessing Mixing Quality of a Poly(1-vinylpyrrolidone-co-vinyl acetate)-TPGS Hot Melt Extrusion
Hot melt extrusion, a process common to the plastic industry, represents a robust technique for preparing amorphous drug-polymer mixtures from lab-scale feasibility assessment to commercial manufacture. Poly(1-vinylpyrrolidone-co-vinyl acetate), a water-soluble, random copolymer of polyvinyl pyrrolidone and polyvinyl acetate commonly used in the pharmaceutical field to prepare amorphous solid dispersions, and D-α-tocopherol polyethylene glycol 1000 succinate (TPGS 1000), a vitamin E derivative often included with the drug in these dispersions to aid in solubilization, were selected as model materials to assess potential phase separation. Various Poly(1-vinylpyrrolidone-co-vinyl acetate)-TPGS compositions were prepared by HME while evaluating the effects of screw speed and quench rate on the extent of mixing for the process. Here, we show that AFM can assess the homogeneity of the extrudates with high spatial resolution and for cases where phase separation is detected, the size and shape of the domains. In addition, an AFM technique known as force spectroscopy was employed to measure the relative differences of mechanical properties of these domains. Finally, modulated differential scanning calorimetry (mDSC) data is provided to complement the AFM data and provide additional insight into the phase behavior of the material prepared by HME.[1]
Increasing screw speed from 300 to 600 rpm resulted in an appearance change of the extrudate, where material was again clear on discharge from the die. the utility of atomic force microscopy to characterize the phase behavior and morphology of solid dispersions comprised of Poly(1-vinylpyrrolidone-co-vinyl acetate), and TPGS 1000 prepared by hot melt extrusion was demonstrated. The effects of processing parameters including screw speed and cooling method were studied as well as the compositional effects on the resulting hot melt extrudate. At 10% by weight loading of TPGS 1000 in Poly(1-vinylpyrrolidone-co-vinyl acetate), a single phase was observed by AFM and mDSC for the screw speed of 600 rpm. However, a screw speed of 300 rpm for the same composition yielded material that was observed to be phase separated by AFM and mDSC. Thus, the slower screw speed resulted in insufficient mixing and yielded a product that was phase separated on exiting the extruder. Rapid cooling via the chilled roll resulted in reduced crystallinity of the phase-separated TPGS as compared to the slower cooled sample. Reducing the TPGS 1000 loading to 5% by weight resulted in single-phase material generated with both screw speeds (300 and 150 rpm) and cooling rates.
Plasdone S630 Ultra—an improved Poly(1-vinylpyrrolidone-co-vinyl acetate)
Trace levels of reactive impurities such as peroxides have been found in many pharmaceutical excipients, including povidones, Poly(1-vinylpyrrolidone-co-vinyl acetate), polyethylene glycol, and polysorbates. The presence of peroxide impurities leads to the degradation of drug products containing oxygen-sensitive active pharmaceutical ingredients (APIs), resulting in decreased product performance, loss in potency, and formation of toxic degradation impurities. Several approaches have been investigated for reducing the generation of peroxides from excipients, including chemical modification of the cross linker; use of enzymes and metals; and supercritical fluid extraction. However, these approaches can limit the usage of excipients in a drug product because of the requirement of additional methods or additives for reducing peroxide concentration. Therefore, novel excipients such as Plasdone™ S630 Ultra (PS630U) Poly(1-vinylpyrrolidone-co-vinyl acetate), in which the initial peroxide levels as well as subsequent peroxide generation during storage can be controlled, were investigated as a choice of excipient for drug product stabilization against oxidative degradation. The molecular weight, particle size and distribution, flowability, and peroxide stability of the new PS630U Poly(1-vinylpyrrolidone-co-vinyl acetate) were improved compared to those of regular Plasdone™ S630 (PS630) copovidone.[2]
The impact of peroxide levels of PS630 and PS630U on oxidative degradation was successfully studied using the HME technique. Melt viscosity results provided insights into the selection of the HME processing temperature of both Poly(1-vinylpyrrolidone-co-vinyl acetate) grades and exhibited similar complex viscosity profiles as a function of temperature. These observations could be attributed to the superior HME processability of PS630U compared to that of regular PS630 copovidone. Thermal characterization and tabletability of both PS630U and PS630 formulations showed comparable Tg and TS profiles, respectively, indicating similar physicochemical characteristics between PS630 and PS630U copovidones. The stability study of the milled extrudates and tablet formulations confirmed that QF is highly sensitive to trace amounts of peroxides in Poly(1-vinylpyrrolidone-co-vinyl acetate), which caused the oxidation of QF to generate N-oxide impurities. The low initial peroxide levels, reduced growth, and peroxide generation in PS630U, as evidenced by low oxidative impurities after 6M stability studies, suggested that PS630U is relatively stable and showed less peroxide generation at ambient and accelerated stability conditions compared to regular PS630. Furthermore, the outcome from this study revealed the applicability of the novel Plasdone™ 630 Ultra for improved drug product stability against oxidative degradation in ASDs developed using the HME technique.
Solid dispersion formulations of megestrol acetate with Poly(1-vinylpyrrolidone-co-vinyl acetate)
In order to enhance the dissolution profile and oral bioavailability of megestrol acetate (MA), solid dispersions of MA (MASDs) were formulated with Poly(1-vinylpyrrolidone-co-vinyl acetate) and crystal sugar as a hydrophilic polymeric carrier and an inert core bead, respectively. Solvent evaporation method and fluidized bed coating technique were employed. MASDs were categorized as crystalline solid dispersion by the characterization of differential scanning calorimetry and X-ray diffraction. The mass-median diameters of MASDs were in a range of 1.4 to 2.6 μm. Based on drug to polymer ratio, MASD (1:1) and (1:2) were considered as optimized formulations, resulting in a smooth-surfaced homogeneously coated layer with enhanced dissolution rate. Dissolution of MASD was gradually increased up to 15 min, after which it reached a plateau. For the initial period, dissolution rates were in the decreasing order of MASD (1:2) ≥ MASD (1:1) > MASD (1:3) > MASD (1:5) > MASD (1:0.5) > MA powder. In the comparative pharmacokinetic study with Megace OS, a reference drug product, MASD (1:1) showed improved bioavailability of over 220% with 2-fold higher C(max) and 30% faster T(max). We conclude that MASD (1:1) is a good candidate for the development of oral solid dosage forms.
References
[1]Lamm MS, DiNunzio J, Khawaja NN, Crocker LS, Pecora A. Assessing Mixing Quality of a Copovidone-TPGS Hot Melt Extrusion Process with Atomic Force Microscopy and Differential Scanning Calorimetry. AAPS PharmSciTech. 2016 Feb;17(1):89-98. doi: 10.1208/s12249-015-0387-9. Epub 2015 Aug 18. PMID: 26283196; PMCID: PMC4766131.
[2]Butreddy A, Sarabu S, Bandari S, Batra A, Lawal K, Chen NN, Bi V, Durig T, Repka MA. Influence of Plasdone? S630 Ultra-an Improved Copovidone on the Processability and Oxidative Degradation of Quetiapine Fumarate Amorphous Solid Dispersions Prepared via Hot-Melt Extrusion Technique. AAPS PharmSciTech. 2021 Jun 28;22(5):196. doi: 10.1208/s12249-021-02069-9. PMID: 34184149; PMCID: PMC8425456.
[3]Hong SW, Lee BS, Park SJ, Jeon HR, Moon KY, Kang MH, Park SH, Choi SU, Song WH, Lee J, Choi YW. Solid dispersion formulations of megestrol acetate with copovidone for enhanced dissolution and oral bioavailability. Arch Pharm Res. 2011 Jan;34(1):127-35. doi: 10.1007/s12272-011-0115-2. Epub 2011 Apr 6. PMID: 21468924.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGENAPOL(R) X-080 (a sclerosing agent) treats lower-leg varicose veins/venous insufficiency and head-neck vascular malformations, with high efficacy/safety.....
Oct 22,2025SurfactantPoly(1-vinylpyrrolidone-co-vinyl acetate)
25086-89-9You may like
Poly(1-vinylpyrrolidone-co-vinyl acetate) manufacturers
- Poly(1-vinylpyrrolidone-co-vinyl acetate)
-

- 2025-11-30
- CAS:25086-89-9
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Poly(1-vinylpyrrolidone-co-vinyl acetate)
-

- $0.00 / 1KG
- 2025-11-29
- CAS:25086-89-9
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Poly(1-vinylpyrrolidone-co-vinyl acetate)
-

- $5.00/ KG
- 2025-11-28
- CAS:25086-89-9
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS