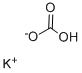
Preparation of potassium bicarbonate
Apr 13,2022
Introduction
Potassium bicarbonate is an inorganic substance with the chemical formula KHCO3. It has a colorless, transparent monoclinic structure[1]. The relative density is 2.17 g / cm ³, It is a stable substance in the air. It is the raw material for the production of potassium carbonate, potassium acetate and potassium arsenite. Potassium bicarbonate is a white solid and belongs to inorganic compounds. The natural mineral is called potash bicarbonate. The ash after plant combustion (commonly known as plant ash) is dissolved in water and then injected with carbon dioxide to form potassium bicarbonate solution. The solution is weakly alkaline. The relative density is 2.17, which is stable in air. Soluble in water and weakly alkaline due to hydrolysis. Aqueous solution and magnesium salt do not precipitate alkaline salt. Insoluble in ethanol. It begins to decompose at 100 ℃ and completely decomposes at 200 ℃, losing carbon dioxide and water to form potassium carbonate.

Picture 1 Potassium bicarbonate powders
Applicaions of potassium bicarbonate
Potassium bicarbonate has a colorless transparent monoclinic structure. The relative density is 2.17, which is stable in air. Soluble in water and weakly alkaline due to hydrolysis. Aqueous solution and magnesium salt do not precipitate alkaline salt. Insoluble in alcohol. It begins to decompose at 100 ℃ and completely decomposes at 200 ℃, losing carbon dioxide and water to form potassium carbonate. It is used as raw material for producing potassium carbonate, potassium acetate and potassium arsenite. Page spraying can be used as fire extinguishing agent for petroleum and chemicals. It can also be used in medicine and baking powder
Potassium bicarbonate comes from a wide range of sources, so it is obtained relatively cheaply. Potassium bicarbonate can be used as acidity regulator, such as medical buffer and wine making additive. A small amount of potassium bicarbonate is added in carbonated drinks to improve taste. Potassium bicarbonate can be used as a fire extinguishing agent. As a special dry powder fire extinguishing agent for the airport, the fire extinguishing effect is twice that of sodium bicarbonate. In agriculture, potassium bicarbonate is used as a soil acidity improver. It is also a permitted pesticide in organic agriculture because of its anti insect and antibacterial effects (such as leaf white mold and apple scab).
Sodium bicarbonate is a common treatment for urine alkalization to promote the excretion of uric acid. In case of metabolic acidosis, patients with chronic kidney disease can also use oral sodium bicarbonate to correct the disorder of acid-base balance in the body
Storage of potassium bicarbonate
Store in ventilated and dry warehouse. It should not be stored in the shed or in the open air. Pay attention to moisture. During transportation, loading and unloading, it shall be protected from rain to ensure that the package is dry and free from damage and pollution. It cannot be stored and transported together with acids. In case of fire, water can be used for rescue.
Preparation of potassium bicarbonate
A solution of potassium hydroxide or carbon dioxide saturated with hydrogen is absorbed to produce 80% potassium carbonate solution.
Carbonization method: the raw material potassium carbonate can be grade III. Earth alkali can also be used as raw material, which contains 40% ~ 60% of potassium carbonate, 10% ~ 15% of potassium sulfate and 3.5% of potassium chloride. Before feeding, organic matter needs to be removed by calcination. Potassium sulfate and potassium chloride can be removed by different solubility. Impurities such as silicon, aluminum and phosphorus can be removed by adding lime milk or magnesium carbonate through pressure filtration. The filtrate is evaporated to prepare potassium carbonate solution, so that the total alkali concentration is 750 ~ 800 g / L (calculated by potassium carbonate) and enters the carbonization tower. When the temperature is above 50 ℃ and the reaction pressure is 0.4MPa, carbon dioxide (concentration is above 30%) is passed for carbonization, and potassium bicarbonate is continuously precipitated with the increase of concentration. After carbonization for 5 ~ 6h, the mother liquor is crystallized, washed, centrifuged and dried at 80 ℃ to prepare the finished product of potassium bicarbonate. Its K2CO3 + CO2 + H2O → 2KHCO3
Ion exchange method: the potassium chloride solution flows through the ion exchange column through the counter current of calcium and magnesium removal to turn the sodium resin (r-na) into potassium resin (r-K). After washing the chloride ion with soft water, the ammonium bicarbonate solution flows downstream through the resin exchange column to obtain the mixed dilute solution of potassium bicarbonate and ammonium bicarbonate. After evaporation and decomposition, most of it is decomposed into potassium carbonate. The solution is sent to the carbonization tower for carbonization to produce potassium bicarbonate, which is then crystallized, separated, washed and dried, The finished product of potassium bicarbonate was prepared. R-Na + KCl → R-K + NaCl; R-K+NHHCO3→R-NH4+KHCO3; 2KHCO3→K2CO3+CO2↑+H2O; K2CO3+CO2+H2O→2KHCO3。The product is prepared by absorbing carbon dioxide from 80% ethanol solution of potassium hydroxide or saturated solution of potassium carbonate. K2CO3+CO2+H2O→2KHCO3。
Reference
1 Nitta I, Tomiie Y, Koo C H. The crystal structure of potassium bicarbonate, KHCO3[J]. Acta Crystallographica, 1952, 5(2): 292-292.
- Related articles
- Related Qustion
- Potassium bicarbonate: The all-rounder of the chemical world Oct 24, 2024
This article popularize the chemistry of potassium bicarbonate in an all-round way and bring you to appreciate its unique style in the chemical world.
4-Aminobutyric acid (GABA) is a compound with the chemical formulaC4H9NO2, is an amino acid.....
Apr 13,2022APISuccinimide is a dicarboximide that is pyrrolidine which is substituted by oxo groups at positions 2 and 5. It is a pyrrolidinone and a dicarboximide.....
Apr 13,2022Organic ChemistryPotassium bicarbonate
298-14-6You may like
Potassium bicarbonate manufacturers
- Potassium bicarbonate
-
- 2025-12-19
- CAS:298-14-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- POTASSIUM BICARBONATE
-

- $10.00 / 1KG
- 2025-12-11
- CAS:298-14-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Potassium bicarbonate
-

- $1200.00 / 1ton
- 2025-11-07
- CAS:298-14-6
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 1000T/M