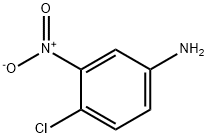
Research on the toxicity of 4-chloro-3-nitroaniline
Jan 12,2024
Unpublished data on the toxicological properties of 4-Chloro-3-nitroaniline (4C3N) indicate that it has an oral LD50 = 336 mg/kg for rats of either sex; it is a slight skin irritant to guinea pig skin and repeated 4C3N application exacerbates the skin reaction. 4C3N is a slight irritant to rabbit eyes and a skin sensitizer for guinea pigs. 4C3N produces only slight methemoglobin in rats. An 200 mg/kg 4C3N oral dose produced 2-5% methemoglobin within 2-4 hr[1].
4-Chloro-3-nitroaniline was given to groups of 20 male and 20 female rats by gavage at doses of 3.6, 18, or 90 mg/kg in a 10% corn oil suspension.
The high dose resulted in reduced body weight gain in males, reduced feed consumption and fluctuating feed consumption in both sexes, slight hemolytic anemia with Heinz bodies in both sexes, enlargement of the spleen due to congestion, hemosiderosis, and increased extramedullary hematopoiesis in both sexes, inflammatory changes in the splenic capsules of two females, hemosiderin pigmentation of the liver in both sexes, bone marrow hyperplasia in both sexes, increased liver weight in females, and testicular atrophy.
The middle dose produced a fluctuating feed consumption pattern in males, Heinz bodies in both sexes, very slight anemia in females, splenic hemosiderosis in males, slightly increased splenic extramedullary hematopoiesis in females, hemosiderin pigmentation of the liver in males, and possibly increased liver weight in females, and inflammatory changes in the splenic capsule of one male.
The low dose produced Heinz bodies in males and possibly females, although the Heinz body counts of females were not statistically significant. The primary toxicologic damage produced by 4-chloro-3-nitroaniline is hemolytic or Heinz body anemia and testicular atrophy. Thus, the toxicity of 4-chloro-3-nitroaniline is similar to that of other aromatic nitro and amino chemicals.
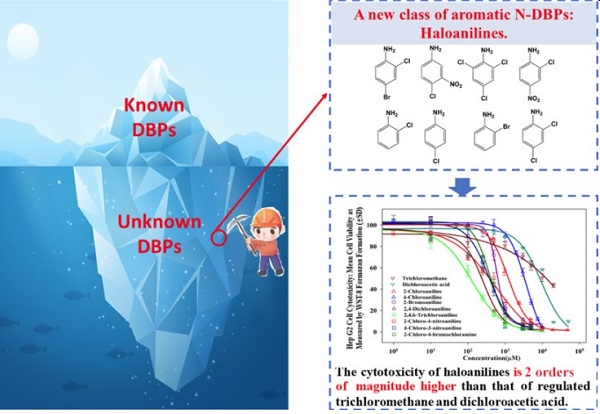
Six members of a new class of aromatic nitrogenous DBPs─2-chloroaniline, 2-bromoaniline, 2,4-dichloroaniline, 2-chloro-4-bromoaniline, 4-chloro-3-nitroaniline, and 2-chloro-4-nitroaniline─are reported as Identifying disinfection byproducts (DBPs) in drinking water. In addition, the EC50 value of 4-chloro-3-nitroaniline is 372.9 μM. Their cytotoxicity was in the following order: 2,4,6-trichloroaniline > 2,4-dichloroaniline > 4-chloro-3-nitroaniline > 2-chloro-4-bromoaniline > 2-chloro-4-nitroaniline > 2-bromoaniline > 2-chloroaniline > 4-chloroaniline ≫ DCAA > TCM[2].
References
[1] O’Donoghue, John L. “Subchronic oral toxicology of 4-chloro-3-nitroaniline in the rat.” Fundamental and Applied Toxicology 6 3 (1986): Pages 551-558.
[2] Di Zhang. “Identification, Occurrence, and Cytotoxicity of Haloanilines: A New Class of Aromatic Nitrogenous Disinfection Byproducts in Chloraminated and Chlorinated Drinking Water.” Environmental Science & Technology 56 7 (2022): 4132–4141.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringFolic acid is the active form of vitamin B9 and occurs naturally in many foods. Folic acid helps the body make healthy red blood cells and is vital during periods of rapid growth, such as pregnancy and foetal development.....
Jan 12,2024Vitamins and Minerals medicines4-Chloro-3-nitroaniline
635-22-3You may like
4-Chloro-3-nitroaniline manufacturers
- 4-Chloro-3-nitroaniline
-

- $1.00 / 1g
- 2020-01-10
- CAS:635-22-3
- Min. Order: 1g
- Purity: 99.0%
- Supply Ability: 100kg