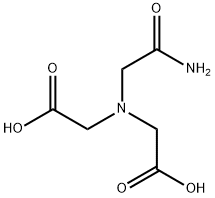
Structural Characteristics and Therapeutic Applications of N-(2-Acetamido)iminodiacetic acid
Dec 3,2025
N-(2-Acetamido)iminodiacetic acid is a white to off-white solid powder under ambient conditions, exhibiting high polarity and excellent chemical stability with moderate solubility in water but poor solubility in ethereal organic solvents. Possessing multiple coordination sites, N-(2-acetamido)iminodiacetic acid can form complexation reactions with various transition metal ions, making it suitable for the preparation of metal ion complexes.

Figure1: Picture of N-(2-Acetamido)iminodiacetic acid
Structural Characteristics
Researchers systematically investigated ternary complexes synthesized from Co(II), Ni(II), and Cu(II) ions with N-(2-acetamido)iminodiacetic acid (disodium salt) and representative di- or tricarboxylic aliphatic acids (succinic, malic, tartaric, and citric acids, abbreviated as H₂L or H₃L). Structural characterization of the obtained complexes was performed using elemental analysis, thermal analysis, infrared and electronic spectroscopy, complemented by conductivity measurements. The Co(II) and Ni(II) complexes were found to adopt an octahedral coordination geometry, whereas the Cu(II) analogues exhibited a tetragonally distorted configuration. Furthermore, the coordination behavior of N-(2-acetamido)iminodiacetic acid was analyzed in relation to the structural characteristics of the carboxylate anions and the metal ion type, revealing significant correlations with the stability trends observed in both binary and mixed-ligand complexes. These findings provide new insights into how the ligand geometry of N-(2-acetamido)iminodiacetic acid influences the overall stability and coordination architecture in these ternary systems. [1]
Thorium Decorporation Agent Preparation
The most effective strategy for mitigating the toxic effects of internal exposure to radiometals in humans is metal-ligand (ML) chelation therapy. Chronic internal exposure to thorium (Th) poses carcinogenic risks and other health hazards, necessitating the development of efficient Th-decorporating agents. In this context, chemical and biological studies were conducted to evaluate N-(2-acetamido)iminodiacetic acid as a comparatively cost-effective, readily available, and biologically safe complexing agent for Th decorporation. In the present work, detailed thermodynamic investigations on the complexation of N-(2-acetamido)iminodiacetic acid with Th(IV) were carried out using potentiometry, calorimetry, electrospray ionization mass spectrometry, and theoretical calculations, followed by biological assessment of its decorporation efficacy. Thermodynamic analyses confirmed the formation of strong Th–ligand complexes that are both enthalpically and entropically stabilized. Density functional theory calculations revealed an unconventional coordination pattern wherein the denticity of N-(2-acetamido)iminodiacetic acid in the ML complex is lower than in ML₂, a phenomenon attributed to hydrogen-bond stabilization in the former, which also correlates with atypical enthalpy trends in the Th–ligand complexes. Biological evaluations using human erythrocytes, whole blood, and lung cells demonstrated excellent cytocompatibility and a significant inhibitory effect on Th-induced hemolysis. The Th-removal capacity of N-(2-acetamido)iminodiacetic acid from erythrocytes, whole blood, and normal lung cells was comparable to that of diethylenetriaminepentaacetate (DTPA), an FDA-approved decorporation agent. This study provides important insights into the thorium complexation behavior of N-(2-acetamido)iminodiacetic acid and its effectiveness in Th decorporation across human biological systems. [2]
Therapeutic Applications
Researchers provides a comprehensive thermodynamic characterization of the complexation between N-(2-acetamido)iminodiacetic acid and Th(IV), alongside an evaluation of its potential for thorium decorporation from human blood and lung cells. Potentiometric and electrospray ionization mass spectrometry analyses confirmed the formation of ML₁, ML₂, and ML₃-type stable complexes between thorium and N-(2-acetamido)iminodiacetic acid. The interaction energetics indicated a reaction driven by both enthalpic and entropic contributions. Theoretical investigations elucidated the Th–ligand coordination at the molecular level, with density functional theory calculations revealing an unconventional increase in denticity from bidentate (ML) to tridentate (ML₂) for N-(2-acetamido)iminodiacetic acid—contrary to steric expectations—attributed to structural stabilization via hydrogen bonding between the amide oxygen and coordinated water molecules in the ML complex. This denticity change correlated with a markedly higher complexation enthalpy for ML₂ relative to ML. Biological assessments demonstrated the ligand's high biocompatibility (up to 200 μM) and significant capacity to inhibit Th-induced hemolysis, performing comparably to the FDA-approved drug DTPA. Ex vivo chelation assays confirmed efficient thorium removal from human erythrocytes, whole blood, and lung cells. Given its efficacy, cost-effectiveness, ready availability, and low toxicity, N-(2-acetamido)iminodiacetic acid warrants further investigation in cellular and animal models for radiological emergency applications, and may also be suitable for thorium sequestration or separation of tetravalent actinides from complex nuclear waste streams. [2]
Reference
[1] M. R. Mahmoud, Divalent Transition Metal Ion Ternary Complexes of N-(2-Acetamido)iminodiacetic Acid and Some Bi- and Tricarboxylic Aliphatic Acids, 2006, 22, 1661-1679.
[2]ShikhaSharma, Combined Thermodynamic, Theoretical, and Biological Study for Investigating N?(2-Acetamido)iminodiacetic Acid as a Potential Thorium Decorporation Agent. Inorg.Chem. 2023, 62,18887?18900.
- Related articles
- Related Qustion
2,4-dichloro-3,5-dimethylphenol is primarily employed in the production of multifunctional care agents and polymeric materials.....
Dec 3,2025APIPhenethyl chloride is synthesized from phenethyl alcohol, used in fine chemicals, and decomposes via HCl elimination to styrene during PVC pyrolysis.....
Dec 3,2025Chemical ReagentsN-(2-Acetamido)iminodiacetic acid
26239-55-4You may like
N-(2-Acetamido)iminodiacetic acid manufacturers
- N-(2-Acetamido)iminodiacetic acid
-

- $0.00 / 1kg
- 2025-12-03
- CAS:26239-55-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- N-(2-Acetamido)iminodiacetic acid
-

- $0.00 / 1KG
- 2025-12-03
- CAS:26239-55-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 3500kg/month
- N-(2-Acetamido)iminodiacetic acid
-

- $1.10 / 1g
- 2025-11-18
- CAS:26239-55-4
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min