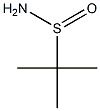Synthesis and Applications of tert-Butanesulfinamide
Jun 16,2025
Introduction
Over the past decade, an ever increasing collection of methods based upon the chiral amine reagent tert-butanesulfinamide (Figure 1) have become some of the most extensively used synthetic approaches for both the discovery and production of drug candidates. Moreover, tert-butanesulfinamide is increasingly being applied across many additional research areas, including the development of agrochemicals, natural product synthesis, and the preparation of chemical tools for a wide range of biological investigations. A number of factors have led to the popularity of tert-butanesulfinamide. From a practical standpoint, either enantiomer of tert-butanesulfinamide is inexpensive to prepare on a large scale in enantiomerically pure form. Indeed, greater than 75 chemical suppliers currently market tert-butanesulfinamide in many cases at reasonable prices (<$1/g for bulk quantities). More importantly, the synthesis steps used to prepare amines from tert-butanesulfinamide are typically robust, straightforward, and broad in scope.[1]Specifically,tert-butanesulfinamides have been applied in the synthesis of enantiopure sulfinylimines, a-branched amines, allylic amines, propargylic amines, α-amino acid and β-amino acid derivatives, 1,2-amino alcohols, 1,3-amino alcohols,1, 2-diamine, 1, 3-diamines, fluorinated amine derivatives, α-organometallic amines,aziridine,and cyclic sulfinamides. In asymmetric catalysis, tert-butanesulfinamides are increasingly being incorporated within chiral ligand and organocatalyst frameworks. Enantiomerically pure tert-butanesulfinamide was original synthesized via asymmetric oxidation of tert-butyl disulfide, followed by nucleophilic displacement with lithium amide.The oxidation product of tert-butyl disulfide, namely, (R)-tert-butyltert-butanethiosulfinate ((R)-1), yields (R)-tert-butanesulfinamide((R)-2) with inversion of configuration.[2]

Synthesis of tert-Butanesulfinamide
An improved synthesis of tert-butanesulfinamide that overcomes the scalability problems of the previous syntheses is described. The key step is the catalytic asymmetric oxidation of the inexpensive di-tert-butyl disulfide starting material. The new homogeneous reaction conditionsutilize an inexpensive chiral ligand prepared in a single step from commercially available cis-1-amino-indan-2-ol. The reaction is performed at a 2.3 M concentration in the practical solvent acetone and can readily be run on a kilogram scale.[3] Danie et al. had reported a new homogeneous oxidation procedure that proceeds efficiently, independent of the reaction scale,and is performed at high concentrations using the inexpensive and relatively nontoxic solvent acetone. In addition, the procedure utilizes an inexpensive ligand that can be prepared as either enantiomer in a single step from commercially available materials. Indeed, the new oxidation procedure proceeds with sufficiently high conversion and fidelity that tert-butanesulfinamide 1 may be prepared by direct addition of lithium amide to the oxidation product 3 without purification of 3 (Scheme 1).The significant improvements to the oxidation procedure are the use of the inexpensive and readily available ligand 5 (Figure 2), the use of the cheap and relatively nontoxic solvent acetone, and the use of only a single purification step. Most importantly, the use of homogeneous reaction conditions eliminates any scale dependence for conversion or enantioselectivity. This new, more efficient route to 3 enables easier, lower cost access to large quantities of tert-butanesulfinamide.


Preparation of tert-butanesulfinyl imines
High-yielding and general methods for the preparation of tert-butanesulfinyl imines are critical to the successful application of tert-butanesulfinamide in the asymmetric synthesis of amines. The authors developed the first direct condensation of sulfinamides with aldehydes and ketones to provide sulfinyl aldiminesand ketimines, respectively. The most straightforward method for the preparation of tert-butane-sulfinyl aldimines is the condensation of aldehydes and tert-butanesulfinamide with CuSO4 as a stoi-chiometric water scavenger and Lewis acid catalyst. Simple filtration and extraction provides thesulfinyl imine in high purity. We have found Ti(OEt)4 to be the ideal water scavenger and Lewis acidfor the preparation of tert-butanesulfinyl ketimines . Ti(OEt)4 is also effective for sterically hindered aldehydes such as pivaldehyde.[4]
Asymmetric synthesis of a-branched amines
α-Branched amines can be obtained by addition of Grignard reagents to N-sulfinyl aldimines in highyields (80–100%) and with high diastereoselectivities (89:11 to 99:1). Broad substrate generalityis observed, with aliphatic and aromatic aldimines and alkyl, aryl, and vinyl Grignards serving as goodcoupling partners. It is notable that even the addition of Grignard reagents to aldimines having α-pro-tons, including the highly acidic arylacetaldimine, proceeds in good yields. A six-membered cyclic transition state with Mg coordinated to the oxygen of the sulfinyl group is consistent with the sense of induction. The proposed cyclic transition state is also consistent with the reaction proceeding with highest selectivities in noncoordinating solvents.
The sulfinyl group is removed from the Grignard addition product by brief treatment with stoi-chiometric quantities of HCl in a protic solvent to provide the desired amine hydrochloride in near quantitative yields. Enantiomerically pure material may be obtained by either crystallizing the amine hydrochloride salt or by first chromatographing or recrystallizing the Grignard addition product prior to sulfinyl group removal. As a second general method for the preparation of α-branched amines that complements the Grignard addition chemistry, they have also developed a one-pot asymmetric synthesis of tert-butane-sulfinyl protected amines from ketones. In this procedure, ketones are condensed with tert-butanesulfinamide using Ti(OEt)4 followed by addition of NaBH4 to the reaction solution at -48°C. The sulfinamide products are obtained in 66–86% yields for the process and with diastereoselectivities ranging from 90:10 to 97:3 for both aryl alkyl and dialkyl ketones. Notably, Ti(OEt)4 not only serves to mediate the imine condensation step, but also serves as a Lewis acid to provide enhanced reduction rates and diastereoselectivities.[4]
References
1.Robak MT, Herbage MA, Ellman JA. Synthesis and applications of tert-butanesulfinamide. Chem Rev. 2010;110(6):3600-3740. doi:10.1021/cr900382t
2.Qiu S, Li G, Lu S, Huang B, Feng Z, Li C. Chiral sulfur compounds studied by Raman optical activity: tert-butanesulfinamide and its precursor tert-butyl tert-butanethiosulfinate. Chirality. 2012;24(9):731-740. doi:10.1002/chir.22038
3.Weix DJ, Ellman JA. Improved synthesis of tert-butanesulfinamide suitable for large-scale production. Org Lett. 2003;5(8):1317-1320. doi:10.1021/ol034254b
4.Ellman J. Applications of tert-butanesulfinamide in the asymmetric synthesis of amines. Pure and Applied Chemistry. 2003;75(1): 39-46. https://doi.org/10.1351/pac200375010039
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGinsenoside Rb1 offers neuroprotection via anti-oxidant/inflammatory effects and aids in cardiovascular health, linked to gut microbiota modulation.....
Jun 17,2025Chinese Herbstert-Butanesulfinamide
146374-27-8You may like
tert-Butanesulfinamide manufacturers
- tert-Butanesulfinamide
-

- $10.00 / 1KG
- 2025-12-03
- CAS:146374-27-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- tert-Butanesulfinamide
-

- $1.10 / 1g
- 2025-11-18
- CAS:146374-27-8
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min
- tert-Butanesulfinamide
-

- $10.70 / 1kg
- 2025-05-26
- CAS:146374-27-8
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 10000kg