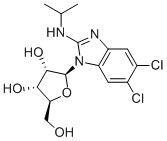
Synthesis and Introduction of Maribavir
Jan 9,2024
Synthesis of Maribavir
Maribavir is synthesised using 4,5-Dichloro-1,2-phenylenediamine as a raw material by chemical reaction. The specific synthesis steps are as follows:
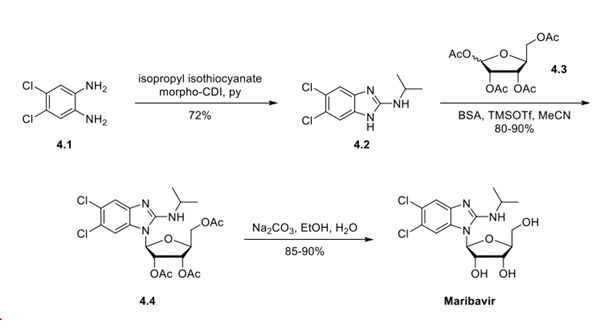
4,5-Dichloro-1,2-phenylenediamine (4.1) was reacted with isopropyl isothiocyanate in pyridine in the presence of a desulfurizing agent, N-cyclohexyl-N′-(2-morpholinoethyl)- carbodiimide methyl-p-toluenesulfonate (morpho-CDI), to generate benzimidazole 4.2 in 72% yield. This was coupled with 1,2,3,5-tetra-O-acetyl-β-L-ribofuranose (4.3) under Vorbruggen conditions (N,O-bis(trimethylsilyl)acetamide, trimethylsilyl trifluoromethanesulfonate) to generate ribofuranoside 4.4 in 80−90% yield. The desired anomer is formed due to the O-acetyl neighboring group's participation during the condensation, following Baker's 1,2-trans rule. Notably, the unnatural L-sugar configuration affects the anti-CMV mechanism of the drug and dramatically improves the drug's pharmacokinetic profile. Deacetylation using sodium carbonate in EtOH/water provided maribavir in 85−90% yield.
Introduction of Maribavir
Maribavir, developed by Takeda Pharmaceuticals, was approved by the USFDA in November 2021 for the treatment of post-transplant cytomegalovirus (CMV) infection/disease that is refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir, or foscarnet in adults and pediatric patients 12 years of age and older and weighing at least 35 kg. While post-transplant CMV infections are common, limited treatments are available. Maribavir is the first and only option for patients with treatment-resistant or refractory CMV. Maribavir is active by the inhibition of CMV protein kinase UL97, preventing human CMV replication. Since maribavir acts via a novel mechanism, it is suitable for patients who have demonstrated resistance to other anti-CMV agents. In an open-label Phase 3 trial, maribavir compared favorably to an active investigator-assigned treatment, with 55.7% versus 23.9% of patients, respectively, reaching the lower limit of quantification of plasma CMV DNA by the end of week 8.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringFluoroiodomethane (CH2FI) has emerged as an easy-to-handle, non-ozone-depleting agent and readily available platform for monofluoromethylation strategies.....
Jan 9,2024Organic reagentsBENZIMIDAVIR
176161-24-3You may like
- Diosgenin:Uses,Functions and Synthesis
Dec 12, 2025
- Biosynthesis of Cyclopamine from Cholesterol
Dec 10, 2025
- Synthesis of ribociclib
Dec 10, 2025
BENZIMIDAVIR manufacturers
- BENZIMIDAVIR
-
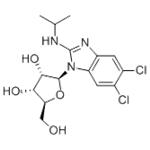
- $0.00 / 1kg
- 2025-12-13
- CAS:176161-24-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Maribavir
-
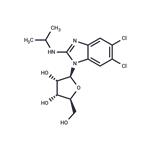
- $31.00 / 1mg
- 2025-12-10
- CAS:176161-24-3
- Min. Order:
- Purity: 100.00%
- Supply Ability: 10g
- BENZIMIDAVIR
-

- $0.00 / 25kg
- 2025-12-01
- CAS:176161-24-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000kg