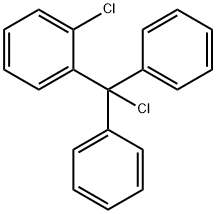
Synthesis and Pharmaceutical applications of 2-Chlorotrityl chloride
Nov 26,2025
2-Chlorotrityl chloride is a highly reactive benzyl chloride derivative that exists as a white solid under ambient conditions, insoluble in water but soluble in organic solvents such as chloroform and ethyl acetate. In the chemical industry, 2-chlorotrityl chloride primarily serves as a pharmaceutical intermediate, with studies reporting its application in the synthesis of the drug molecule clotrimazole. Clotrimazole is a broad-spectrum antifungal agent demonstrating potent activity against various fungi, particularly Candida albicans, through mechanisms involving inhibition of fungal cell membrane synthesis and disruption of metabolic processes. This medication exhibits efficacy against both superficial and certain systemic fungal infections, and is clinically administered topically for treating dermatomycoses including tinea pedis, tinea corporis, otomycosis, and vaginal mycosis.

Figure1: Picture of 2-Chlorotrityl chloride
Synthesis
The synthesis of 2-Chlorotrityl chloride involves a three-step sequence: esterification of o-chlorobenzoic acid with ethanol to form ethyl o-chlorobenzoate, followed by a Grignard reaction with phenylmagnesium bromide yielding (2-chlorophenyl)diphenylmethanol magnesium salt, and final chlorination after sulfuric acid hydrolysis to obtain the target compound 2-Chlorotrityl chloride.
Esterification reaction
The esterification of 2-chlorotrityl chloride resin with Fmoc-amino acids in the presence of DIEA is studied under various conditions. High esterification yields are obtained using 0.6 equiv. Fmoc-amino acid/mmol resin in DCM or DCE, in 25 min, at room temperature. The reaction proceeds without by product formation even in the case of Fmoc-Asn and Fmoc-Gln. The quantitative and easy cleavage of amino acids and peptides from 2-chlorotrityl resin, by using AcOH/TFE/DCM mixtures, is accomplished within 15-60 min at room temperature, while t-butyl type protecting groups remain unaffected. Under these exceptionally mild conditions 2-chlorotrityl cations generated during the cleavage of amino acids and peptides from resin do not attack the nucleophilic side chains of Trp. Met, and Tyr. [1]
Peptide synthesis
Fmoc/tBu-protected peptides, synthesized on 2-Chlorotrityl chloride, have been quantitatively esterified by treatment with 2-Chlorotrityl chloride (Clt-chloride) and diisopropylethylamine (DIPEA). The obtained peptide Clt-esters have been deprotected at the Nα-function and subsequently condensed in solution to larger peptides. The Clt-function of the obtained products could be selectively removed by treatment with acetic acid. The application of 2-chlorotrityl chloride resin to the preparation of C-terminal peptide N-alkylamides was also studied. Leuprorelin,an ethylamide peptide,was used as a model.Fully-protected peptide was first assembled with this resin,and then amidated by HBTU/HOBt/DIEA/ethylamine(1:1:1:10,mole ratio) at the C-terminal for only 5 min at 25(°C) and then deprotected at the side-chain immediately.In this method,the purification step between ethylamination and deprotection was avoided,and the efficiency of solid-phase peptide synthesis was inherited. [2-3]
Solid-phase synthesis of peptide
Solid-phase synthesis serves as a fundamental strategy for peptide construction and is equally applicable to the preparation of peptidomimetic organic molecules. Although multiple routes exist for synthesizing peptidomimetics, this approach demonstrates superior efficiency over conventional chemical methods when facing constrained synthesis timelines and requiring minimal product quantities for analytical purposes. Prior to constructing peptidomimetics using 2-Chlorotrityl chloride resin, multiple factors require comprehensive consideration. The most notable advantage of 2-Chlorotrityl chloride-based methodology lies in eliminating tedious purification columns after each synthetic step—resin beads merely undergo washing with excess solvent and concentration before proceeding to subsequent coupling reactions, thereby significantly accelerating the synthetic workflow. Unlike standard peptide synthesis, the entire process mandates the use of anhydrous solvents.
Pharmaceutical applications
In the chemical industry, 2-chlorotrityl chloride functions as a key pharmaceutical intermediate, with documented applications in the synthesis of bioactive molecules including the antifungal agent clotrimazole. The anticancer peptaibol culicinin D was synthesized through a novel pathway featuring optimized attachment of C-terminal amino alcohol building blocks to 2-chlorotrityl chloride resin. A model system with Fmoc-alaninol substituting the unusual APAE block demonstrated efficient resin loading via N-anchoring and intramolecular O-N acyl shift.
Reference
[1] Chatzi K B O , Gatos D , Stavropoulos G .2‐Chlorotrityl chloride resin[J].Int.j.peptide Protein Res, 1991, 37:513-520.
[2] Tzavara H C .Application of 2-chlorotrityl chloride in convergent peptide synthesis[J].Tetrahedron Letters, 1995.
[3] LI Pei-hua.Application of 2-Chlorotrityl Chloride Resin to the Solid Phase Synthesis of C-terminal Peptide N-alkylamides[J].Chemical World, 2007.
- Related articles
- Related Qustion
Tall Oil Fatty Acid (TOFA) is defined as a fatty acid-enriched compound obtained through the fractionation and purification of tall oil.....
Nov 26,2025APIAngiotensin II human has been identified as a pivotal component in locally active renin-angiotensin systems across multiple tissues.....
Nov 26,2025API2-Chlorotrityl chloride
42074-68-0You may like
2-Chlorotrityl chloride manufacturers
- 2-Chlorotrityl chloride
-

- $990.00 / 1ton
- 2025-12-16
- CAS:42074-68-0
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 5000
- 2-CHLOROTRITYL CHLORIDE
-

- $10.00 / 1KG
- 2025-12-11
- CAS:42074-68-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 2-CHLOROTRITYL CHLORIDE
-

- $1300.00 / 1kg
- 2025-09-26
- CAS:42074-68-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons