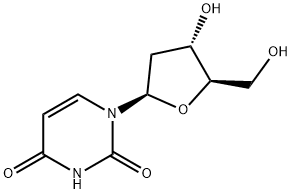
Synthesis and Structural Properties of 2'-Deoxyuridine
Dec 15,2025
2'-Deoxyuridine, a naturally occurring deoxynucleoside, presents as a white to off-white solid powder under ambient conditions and exhibits distinctive physiological activity, characterized by its poor solubility in aqueous media but appreciable solubility in alcoholic organic solvents and dimethyl sulfoxide. Primarily utilized as a biosynthetic intermediate, 2'-Deoxyuridine serves as a key precursor for the preparation of antiviral nucleoside analogues and molecular markers, exemplified by its role as a starting material in the synthesis of biologically significant compounds such as 8-bromo-2'-deoxyguanosine and 8-hydroxy-2'-deoxyguanosine.

Figure1: Picture of 2'-deoxyuridine
Structural Properties
The interaction of 2'-deoxyuridine with human serum albumin (HSA) was systematically investigated through fluorescence spectroscopy coupled with molecular modeling under simulated physiological conditions across three temperature levels, with analysis confirming a static quenching mechanism based on the fluorescence measurements. According to the thermodynamic parameters derived for 2'-deoxyuridine, the enthalpy change (ΔH) and entropy change (ΔS) were determined via the Vant’Hoff equation as 18.87 kJ/mol and 24.00 J/(mol·K), respectively, indicating that hydrophobic interactions represent the dominant intermolecular forces responsible for complex stabilization. Experimental observations showed strong consistency with molecular modeling predictions, and the influence of various common ions on the binding constants was additionally examined at ambient temperature. The investigation of 2'-deoxyuridine interaction with human serum albumin (HSA) under simulated physiological conditions at 27°C demonstrated that coexisting ions significantly influence their binding affinity, as the presence of trace metal ions (including S, P, Cu, and Mn naturally present in HSA) reduced the binding constants between 2'-deoxyuridine and the protein. This competitive binding mechanism not only shortens the plasma circulation half-life of the nucleoside drug but also enhances its maximum therapeutic effectiveness, confirming that metal-protein interactions directly modulate the drug-albumin binding dynamics. Molecular modeling was employed to improve understanding of the interaction of 2'-Deoxyuridine and HSA. From the 3-D structure of crystalline albumin, it is known that HSA comprises three homologous domains. [1]
Synthesis
Method 1
2'-Deoxyuridine exerts its therapeutic effects by interfering with or directly acting upon nucleic acid metabolic pathways, thereby inhibiting the biosynthesis of proteins and nucleic acids, which establishes its critical role in both antiviral and antineoplastic chemotherapeutic regimens. While chemical synthesis offers shorter production cycles for 2'-Deoxyuridine, this method suffers from significant limitations including high raw material costs, substantial toxicity concerns, and poor stereoselectivity that typically results in terminal product mixtures containing both α- and β-nucleoside isomers; in contrast, enzymatic synthesis provides excellent stereochemical control albeit with extended processing durations.
Method 2
Nucleosides and their analogs, recognized for their significant antitumor, antiviral, and antibacterial activities, hold an irreplaceable position in clinical practice, rendering their synthetic approaches a continuing research priority. In this work, a hybrid chemoenzymatic strategy was developed for the efficient production of 2'-Deoxyuridine, beginning with 3',5'-O-di-p-chlorobenzoyl-2-deoxy-D-ribose-1-chloride and proceeding through a crystallization-induced asymmetric transformation to furnish the key intermediate 2'-deoxy-α-D-ribose-1-phosphate. Subsequent enzymatic coupling, catalyzed by Escherichia coli nucleoside phosphorylase in the presence of uracil, enabled the direct and selective formation of 2'-Deoxyuridine. Through systematic process optimization, the following conditions were identified as optimal: (1) for the ditributylamine salt of 3',5'-O-di-p-chlorobenzoyl-2-deoxy-α-D-ribose-1-phosphate, a 23 h reaction using molar ratios of ribose-1-chloride:phosphate:tributylamine = 1:3:3 and ribose-1-chloride:acetonitrile = 19:2.3; (2) for the corresponding dicyclohexylamine salt, a 1 h reaction at 0 °C with a 1:3 ratio of ribose-1-chloride to cyclohexylamine, affording 94.53% yield and 95.68% purity; (3) for 2-deoxy-α-D-ribose-1-phosphate dicyclohexylamine salt, treatment with 3% cyclohexylamine in methanol at 45 °C for 36 h, giving 82.69% yield; and (4) for the final synthesis of 2'-Deoxyuridine, reaction with 15 mmol/L uracil at a 1:3 molar ratio of ribose-1-phosphate to uracil, pH 7.0, 40 mg/L cell concentration, 60 °C, and 6 h, achieving 42.32% conversion. This integrated route effectively combines the merits of chemical and enzymatic methods, minimizes the use of hazardous reagents, overcomes the stereoselectivity constraints inherent in conventional synthesis, and operates under mild conditions with reduced processing time, underscoring the broad utility of this optimized synthesis of 2'-Deoxyuridine for both fundamental and applied purposes. [2]
Reference
[1] Fengling Cui, Characterization of the interaction between 20-deoxyuridine and human serum albumin, Carbohydrate Research 2009, 344:642–647.
[2] Peng M H. Chemo-enzymatic synthesis of 2'-deoxyuridine[D]. Zhejiang University of Technology, 2012.
- Related articles
- Related Qustion
γ-Valerolactone is a valuable chemical intermediate that can be obtained by catalytic reduction of levulinic acid or alkyl levulinates.....
Dec 15,2025Organic Synthesis Intermediate1H,1H,2H,2H-Perfluorooctanesulphonic acid serves as a crucial intermediate in organic fluorine synthesis, advanced chemical synthesis.....
Dec 15,2025API2'-Deoxyuridine
951-78-0You may like
- 2'-Deoxyuridine
-

- $0.00 / 1Kg/Bag
- 2025-12-15
- CAS:951-78-0
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 100KG
- 2'-Deoxyuridine
-
- $30.00 / 50mg
- 2025-12-15
- CAS:951-78-0
- Min. Order:
- Purity: 99.92%
- Supply Ability: 10g
- 2'-Deoxyuridine
-
- $10.00 / 1Kg
- 2025-12-15
- CAS:951-78-0
- Min. Order: 1Kg
- Purity: 98%
- Supply Ability: 20Ton