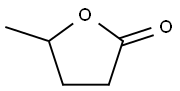
γ-Valerolactone:synthesis and chemical recovery
Dec 15,2025
Introduction
γ-Valerolactone (GVL;Figure 1) is an important chemical and it can be used to produce liquid fuels, as a building block for polymers, an intermediate in the fine chemicals synthesis,a solvent, and a flavoring agent.

Physical data[2]
Molecular Formula: C₅H₈O₂;Molecular Weight: 100.11
Boiling Point: 191.57℃ (EPI Suite)
Flash Point: > 93℃ (GHS), > 200℉; CC (FMA Database)
Log KOW: 0.11 (EPI Suite)
Melting Point: −34.29℃ (EPI Suite)
Water Solubility: 93810 mg/L (EPI Suite)
Specific Gravity: 1.050 (FMA Database)
Vapor Pressure: 1.46E-10 mmHg @ 20℃(EPI Suite v4.0), 0.3 mmHg 20℃ (FMA Database), 3.76e-010 mmHg @25℃(EPI Suite)
UV Spectra: No significant absorbance between 290 and 700 nm;molar absorption coefficient is below the benchmark (1000 L mol−1∙cm−1)
Appearance/Organoleptic: Colorless oily liquid. Warm, sweet,hay- and tobacco-like, herbaceous odor of moderate to poor tenacity. Very sweet, warm-herbaceous, mildly spicy taste at concentrations lower than 100 ppm. The material has a considerable range of "pleasant concentration" and the upper limit for pleasant taste impression is very high, but the taste effect and power is not very great.
Synthesis of γ-Valerolactone from Carbohydrates or Lignocelluloses
There is a growing interest in the synthesis of γ-valerolactone in recent years. One of the most effective methods for the production of γ-valerolactone is the catalytic hydrogenation of levulinic acid (LA), or alkyl levulinates(AL), and several reviews documented the synthesis of γ-Valerolactone from LA or AL well. Despite the successful results in the synthesis of γ-valerolactone from LA or AL, it should be noted that the large-scale synthesis of γ-valerolactone is limited to the supply of the feedstock of LA and AL. As shown in Scheme 1, LA and AL can be obtained from carbohydrates or lignocelluloses through hydrolysis or alcoholysis in water or alcohol in the presence of acid catalysts. Conversion of carbohydrates directly into γ-valerolactone without isolation of the intermediate LA could provide a means of producing γ-valerolactone with minimal processing steps, although this methodology requires the combination of both acid and hydrogenation catalysts.[1] The current methods in the synthesis of γ-valerolactone from carbohydrates or lignocelluloses were introduced.
One-pot conversion of fructose into γ-valerolactone
Qi and Horvath studied the one- pot–two step transformation of fructose into γ-valerolactone using γ-valerolactone as solvent. The first step included the dehydration of fructose into HMF followed by rehydration of HMF to LA in the presence of H2SO4 as catalyst. Then, the reduction of LA into 4-hydroxyvalericacid (4-HVA) was catalyzed using a homogenous Ru catalyst(Shvo catalysts), which was the key step of the one-pot conversion of fructose into γ-valerolactone. 4-HVA readily underwent ring closurevia dehydration to from γ-valerolactone. These steps were confirmed by13C labeling of the fructose used as the feedstock. The use of γ-valerolactone as the solvent opened up the opportunity to develop an attractive and green process for the production of GVL by avoiding separation of solvent and product.[1]
One-pot conversion of glucose-based carbohydrates into γ-valerolactone
Compared to fructose, glucose-based carbohydrates are the preferred feedstock for the synthesis of γ-valerolactone as they are abundant and inexpensive. Therefore, considerable interest has been paid on the synthesis of γ-valerolactone from glucose-based carbohydrates. To improve the overall yield of GVL, a one pot–two step method was mainly applied for the transformation of glucose-based carbohydrates into GVL using homogeneous or heterogeneous metal catalysts.[1]
Chemical Recovery of gamma-Valerolactone/Water Biorefinery
Lê et al. introduced the optimization of the pulping conditions and propose different chemical recovery options for a proven biorefinery concept based on γ-valerolactone (GVL)/water fractionation. The pulping process has been optimized whereby the liquor-to-wood (L:W) ratio could be reduced to 3 L/kg without compromising the pulp properties as raw material for textile fibers production. The recovery of the pulping solvent was performed through combinations of lignin precipitation by water addition, distillation at reduced pressure, and liquid CO2 extraction. With a two-step lignin precipitation coupled with vacuum distillation, more than 90% of lignin and γ-valerolactone could be recovered from the spent liquor. However, a significant part of γ-valerolactone remained unrecoverable in the residue, which was a highly viscous liquid with complicated phase behavior. The recovery by lignin precipitation combined with liquid CO2 extraction could recover more than 85% γ-valerolactone and 90% lignin without forming any problematic residue as in the distillation process. The remaining γ-valerolactone remained in the raffinate containing a low amount of lignin and other compounds, which can be further processed to isolate the γ-valerolactone and improve the recovery rate.[3]
References
[1] Zhang Z. Synthesis of γ-Valerolactone from Carbohydrates and its Applications. ChemSusChem. 2016;9(2):156-171. doi:10.1002/cssc.201501089
[2] Api AM, Belmonte F, Belsito D, et al. RIFM fragrance ingredient safety assessment, γ-valerolactone, CAS Registry Number 108-29-2. Food Chem Toxicol. 2019;134 Suppl 2:110950. doi:10.1016/j.fct.2019.110950
[3] Lê HQ, Pokki JP, Borrega M, Uusi-Kyyny P, Alopaeus V, Sixta H. Chemical Recovery of γ-Valerolactone/Water Biorefinery. Ind Eng Chem Res. 2018;57(44):15147-15158. doi:10.1021/acs.iecr.8b03723
- Related articles
- Related Qustion
Methyl 3-amino-4-methylbenzoate is synthesized via electroreduction and has coplanar substituents with intramolecular hydrogen bonding.....
Dec 15,2025Chemical MaterialsPrimarily utilized as a biosynthetic intermediate, 2'-Deoxyuridine serves as a key precursor for the preparation of antiviral nucleoside analogues.....
Dec 15,2025APIγ-Valerolactone
108-29-2You may like
- gamma-Valerolactone
-
- 2025-12-15
- CAS:108-29-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- gamma-Valerolactone
-
- $74.00 / 1kg
- 2025-12-15
- CAS:108-29-2
- Min. Order: 1kg
- Purity: 0.98
- Supply Ability: 100kg
- gamma-Valerolactone
-

- $2.00 / 1kg
- 2025-12-15
- CAS:108-29-2
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 500mt/year