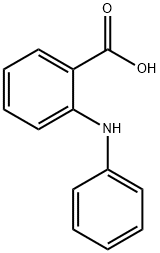
Synthesis of N-phenylanthranilic Acid and Its Related Toxicity and Biomarker Studies
May 8,2025
N-phenylanthranilic Acid, an important intermediate for acridine compounds, has common synthetic methods in the prior art that use toxic substances like nitrobenzene and lead powder or are affected by catalyst types with low yields and high costs.

Synthesis of N-phenylanthranilic Acid
N-phenylanthranilic Acid, also known as vanadium reagent, is an important intermediate for the synthesis of acridine compounds. At present, the common synthetic method of N-phenylanthranilic Acid in the prior art mainly uses anthranilic acid and halogenated benzene to react, under the conditions of lead powder and nitrobenzene, N-phenylamino benzoic acid. However, this method still has some shortcomings. For example, nitrobenzene and lead powder are toxic and harmful substances, which exert great pressure on the human body and the environment, and the reaction yield is low. Others use the Ullmann condensation method, using o-halobenzoic acid and aniline as the main raw materials, using copper-containing catalysts to catalyze the reaction to obtain N-phenylanthranilic Acid, and the yield is greatly affected by the types of catalysts. The use also increases the production cost, which is not conducive to industrial production. In order to solve the disadvantages of low yield, high cost, toxic and harmful effects in the prior art N-phenylanthranilic Acid synthesis method, the present invention provides a harmless, low-cost N-phenylanthranilic Acid The synthesis method of using a one-pot process, without using a catalyst, with 2-chlorobenzonitrile and aniline in a solvent for reflux reaction, and then continue the reflux reaction in the water phase, after neutralization with dilute hydrochloric acid, The target product N-phenylanthranilic Acid is obtained.[1]
A method for synthesizing N-phenylanthranilic Acid, including the following steps: (1)Dissolve 1.0 mol 2-bromobenzonitrile and 1.2 mol aniline in 500 mL of dried solvent DMSO, then add activated 0.1 mol K2CO3 and activated 3.0 mol NaOH under stirring, and perform a reflux reaction with heating, and the reaction time is 2.5h;The activation is to microwave the anhydrous K2CO3 and solid NaOH for 10-15 minutes before use to remove surface moisture and gas, release its effective surface area, and improve its reaction activity.(2)After the reflux reaction is completed, 450 mL of solvent DMSO is recovered under reduced pressure, and then the remaining liquid is cooled to 25° C., 600 mL of water is added, heated to boiling, and a second reflux reaction is performed for 1.5 h;(3)After the second reflux reaction in step (2), the reaction solution was cooled to room temperature, dichloromethane was added to extract excess aniline, diluted hydrochloric acid was added to the aqueous phase while stirring to neutralize the pH to 3-4, the solid was precipitated, and then filtered, Through crystallization, 194.59 g of N-phenylanthranilic Acid as the target product was obtained, with a yield of 91.0% and a content of 99.7%.It can be seen that the present invention uses the cyano group in 2-chlorobenzonitrile to have higher reactivity without requiring a catalyst, and successfully prepares high purity (above 99%) and high yield (total yield of over 90%) The N-phenylanthranilic Acid. In addition, the cost of raw materials used is low, the synthesis process is simple to operate, the reaction is mild and easy to control, and the target product can be obtained through simple post-processing steps. It can meet the needs of industrial production.
Biomarker Studies
N-phenylanthranilic acid is a chloride channel blocker that causes renal papillary necrosis in rats. Studies were conducted in two strains of male rats to evaluate novel biomarkers of nephrotoxicity. Han-Wistar rats were given daily oral doses of 50, 350, or up to 700 mg/kg/day of N-phenylanthranilic acid, and Sprague-Dawley rats were given 50 or 400 mg/kg/day of N-phenylanthranilic acid. Rats were euthanized on days 8 and 15. The candidate kidney injury biomarkers renal papillary antigen-1 (RPA-1, for collecting duct injury), clusterin (for general kidney injury), α-glutathione-S-transferase (a proximal tubular marker), and µ-glutathione-S-transferase (a distal tubular marker) were measured in urine by enzyme immunoassay. Characteristic degeneration and necrosis of the collecting duct and renal papilla were observed in Han-Wistar rats at the high dose on day 8 and at the mid and high doses on day 15, and in Sprague-Dawley rats given the high dose on days 8 and 15. Increases in urinary RPA-1, and to a lesser extent urine clusterin, were generally associated with the presence of collecting duct injury and were more sensitive than BUN and serum creatinine. On the other hand, decreases in α-glutathione-S-transferase without proximal tubule lesions in both strains and decreases in µ-glutathione-S-transferase in Sprague-Dawley rats only were not associated with morphological proximal or distal tubule abnormalities, so both were of less utility. It was concluded that RPA-1 is a new biomarker with utility in the detection of collecting duct injury in papillary necrosis in male rats.[2]
A signal transduction kinase inhibitor and N-phenylanthranilic acid
Biomarkers of nephrotoxicity range from plasma and urine biochemistry, enzymic assays for brush border and lysosomal markers plus new protein markers by immunoassay. Because of the complexity of the nephron and regional sensitivity to xenobiotics, it is important to co-localise sites of marker release with pathological lesions. Han Wistar rats were treated p.o.for up to 14 days with compounds causing selective nephrotoxicity. Compounds used were cyclosporin A ,a signal transduction inhibitor and N-phenylanthranylic acid (NPAA). Plasma and urine was collected for biochemistry and urinalysis (including proteomics and metabonomics) and at termination kidneys were fixed for standard H&E pathology and immunohistochemistry examinations for D28 k calbindin, calmodulin, phospho-erk, Cox 1, Cox 2 and other markers. Cyclosporin A treatment caused injury to the thick ascending limb (TAL) of the nephron and was associated with a down-regulation of calbindin protein expression in cortical distal tubules (mean score 75% reduction) and TALs (21% reduction). Inhibition of signal transduction used p-erk as a downstream marker of activity. P-erk was highly expressed in the collecting ducts and inhibition of signalling caused a 39% reduction in IHC score. There was no evidence of direct renal injury by there was a hypercalcaemia (9% increase) and hyperphosphataemia (24% increase) at 24 hrs post-dose and metastatic calcification by 7 days. N-phenylanthranilic Acid treatment caused renal papillary necrosis in some treated rats (sometimes unilateral) with some secondary dilation of distal tubules. Unlike NSAID treatment, there was no evidence of Cox 1 or 2 dysregulation on IHC and the Cox1 positive interstitial cells did not loose integrity before the onset of necrosis. There were a number of urinary proteomic and metabonomic alterations which are being characterised. The 3 model nephrotoxicants studied demonstrated the linkage of protein expression on IHC to nephron segment-specific sites as important for urinary biomarker validation and linkage to mechanisms.[3]
References
[1]SHANGHAI WOKAI BIOTECHNOLOGY - CN112142610, 2020, A
[2]Betton, Graham R et al. “Biomarkers of collecting duct injury in Han-Wistar and Sprague-Dawley rats treated with N-phenylanthranilic Acid.” Toxicologic pathology vol. 40,4 (2012): 682-94. doi:10.1177/0192623311436174
[3]Betton, Graham R et al. “Protein biomarkers of nephrotoxicity; a review and findings with cyclosporin A, a signal transduction kinase inhibitor and N-phenylanthranilic acid.” Cancer biomarkers : section A of Disease markers vol. 1,1 (2005): 59-67. doi:10.3233/cbm-2005-1107
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAmiodarone hydrochloride is a class III antiarrhythmic medication that is commonly used for the treatment of atrial arrhythmias and ventricular arrhythmias.....
May 8,2025DrugsN-Phenylanthranilic acid
91-40-7You may like
N-Phenylanthranilic acid manufacturers
- fenamic acid
-

- $1.00 / 500g
- 2025-12-13
- CAS:91-40-7
- Min. Order: 300g
- Purity: 99.8%
- Supply Ability: 20 TONS
- Fenamic acid
-
- $78.00 / 500mg
- 2025-12-05
- CAS:91-40-7
- Min. Order:
- Purity: 99.61%
- Supply Ability: 10g
- N-Phenylanthranilic acid
-
- $1.10 / 1g
- 2025-11-18
- CAS:91-40-7
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons